The early detection of a species outside of its previous known distribution, including both native and invaded areas, triggers a series of cascading questions as to (1) the source of propagules (i.e., the donor region), (2) the mechanism by which they were transported and released into the recipient region, (3) the probability that the species will become established and finally (4) the possible impacts it will have. Here we have provided strong evidence for the first two questions and intriguing observations with regards to the latter two.
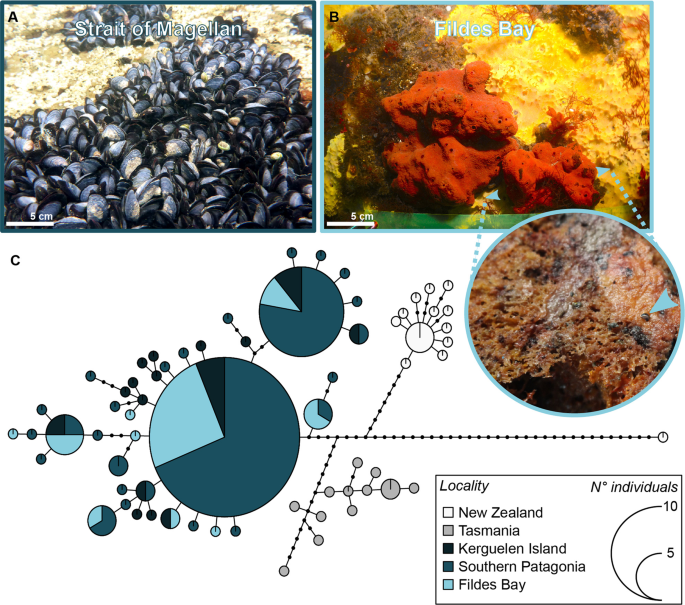
With regards to the source of the mussels that we discovered, we were able to assign all sequenced individuals to the southern hemisphere lineage of Mytilus sp., and more specifically to the S1 clade20. Although there is no consensus for the species name of this clade and it is not the scope of this study to deal with taxonomic questions, we suggest that this clade S1 could be referred to as Mytilus cf. platensis d’Orbigny, 184221,22,23. Due to the relatively long planktotrophic larval period for mussels (up to 6 weeks24), and rafting ability25, mytilids would certainly have the potential to naturally disperse this distance. Indeed, in the late Tertiary era this clade was able to spread 3500 km to the Kerguelen Islands in the southern Indian Ocean, likely aided by strong eastward currents23,26,27. However, the position and strength of the Antarctic Polar Front was quite different at that time20,23, and it now represents a formidable oceanic barrier to poleward migration from South America and nearby islands8,10. For instance, passive dispersal from these locations towards islands of the Antarctic Peninsula likely lasts 1 to 3 years27, which may explain the absence or paucity of living non-indigenous biota observed upon biodegradable rafts (kelp) reaching Antarctic shores13,27.
Although a number of vectors can transport NIS propagules, the limited presence and activities of humans in the Antarctic make ship traffic the most likely vector12 for the mussels that we observed, especially given that most ship traffic either originates or passes through the most probable source population, that of southern Patagonia. However, the exact mechanism is not obvious as mussels can be transported either as adults attached to the hull, especially in the sea chest where fouling can be higher14, or as larvae in ballast water28. If ballast water was the mechanism, then larvae were certainly partially developed when released, but still would have had to complete development, settle and metamorphose. However, it is technically illegal to release ballast water in the nearshore environments of Antarctic (IMO Ballast Water Management Convention, Resolution MEPC.163(56)), which supports the idea that the juveniles were instead the result of adults spawning after the arrival of a fouled vessel29, the probability of which being exacerbated during the warmer period (our results, Fig. 2). If true, then it would mean that at least some of the resulting zygotes were able to complete the entire larval phase as well as settle and metamorphose. Given that the presence of adult Mytilus spp. on ships visiting Antarctica has already been repeatedly documented14 (specimens originating from South Africa were identified as M. galloprovincialis although the authors apparently did not conduct genetic analyses) in contrary to other vectors (e.g. plastic and kelp rafts)13, but see25, we suggest that this latter mechanism was the probable means by which the mussels we observed were released into Antarctic waters.
Regardless of the precise mechanism, the presence of juvenile mussels in Antarctica clearly demonstrates that the initial steps in the invasion process17, namely the transport and release of propagules of non-indigenous species, are occurring in this isolated ecosystem. Moreover, it shows that larvae are able to survive and recruit in this new system and demonstrates that environmental filters (e.g., mismatch between donor and recipient environments17) did not prevent key early phases of the life cycle (e.g., larval development, settlement and metamorphosis, and early juvenile growth) from occurring. This finding is remarkable as there is to the best of our knowledge no indication in the literature that mussel larval development can occur at the consistently low temperatures that characterize Antarctica. Following the mussel settlement herein reported, two key questions remain: will they stay, and if they do, what impact will they have?
“Staying” in this context means establishing a self-sustaining population, which would require both further individual growth and reproduction (gonad development and gametogenesis). Studies of Arctic populations show that gametogenesis and spawning can occur in populations of mytilid mussels at the low temperatures that we recorded in this study30,31, and, as argued above, the presence of juveniles in several locations far from ship activity is strong evidence for a successful passage from fertilization through larval development to settlement and metamorphosis in these very low temperatures. Finally, growth clearly has occurred as the observed shell length of 2 mm is far larger than the size at settlement for M. platensis recruits32. Information from this study demonstrates that the early parts of the mussel’s life cycle could be completed in Antarctic waters, which is consistent with observations of populations of Mytilus elsewhere where low temperatures are normal (e.g. as experienced by intertidal mussels in the Strait of Magellan, Fig. 2), albeit not as continuously low as recorded in Fildes Bay (i.e., <2 °C). However, at the time of this writing, we cannot ascertain the fate of the Fildes Bay mussel population, as the austral summer campaign of 2020 is still ongoing. Preliminary sampling and examination within the very same subtidal habitats suggest that this population has gone extinct locally, although further work in this area is, however, clearly needed to assess the risk of establishment in this environment.
Successful completion of the life cycle is a necessary but not sufficient condition for the establishment of a NIS as demographic limitations (e.g. Allee effect) are also a consideration. This concern is especially relevant to marine invertebrates like mussels that have separate sexes, external fertilization and long planktonic larval periods. This suite of characteristics leads to the dilution of gametes and larvae, which can limit rates of fertilization and recruitment, respectively, and together reduce the probability of establishing a self-sustaining population33. Indeed, several past cases of the appearance of mussel populations outside their known range illustrate the precarious nature of the invasion process. One example was the brief appearance of the temperate species M. galloprovincialis in a subtropical location (Hawaii, USA) where ship-fouling mussels spawned and recruited within a harbor but did not persist29. Another example was the appearance of Mytilus cf. edulis34 in Svalbard (Norway) due to an extreme alteration of oceanic circulation35. The first recruitment was estimated to have occurred in 2002, and subsequent sampling in 2014 confirmed the persistence of this population – likely aided by further inoculation events34. These contrasting examples demonstrate both the ephemeral nature of populations established outside their existing range as well as the need for both early detection efforts and frequent monitoring to ascertain the conditions under which such invasions succeed or fail.
Mussel invasions have been frequent worldwide. Impacts have been varied, but given the general importance of mussels in structuring benthic marine and freshwater communities36, the potential is enormous. One peculiarity of the present case is the occurrence of mussels associated with biogenic structures, such as sponges. Should the mussel remain a rare part of this cryptofauna, its visible impact will likely be negligible. In contrast, should this ecological niche provide a beachhead for spread into other habitats (e.g. notably in the intertidal, which have not presently been sampled but where densest mussel populations are commonly found elsewhere), then impacts could increase. Although the initial steps of colonization may be hampered by a loss of facilitating taxa (e.g. barnacles), Mytilus have the potential to spread in a broad arrays of environments and habitats, ranging from diversified submerged moving vectors25,37 to depauperate and ice-influenced intertidal shores34. At the extreme, the formation of extensive beds as observed in both indigenous and invaded regions38 could have major impacts on native biodiversity, food web dynamics and ecosystem processes (e.g., nutrient dynamics)3,39.
Lurking behind this discussion are two factors that will continue to redefine the probability of NIS establishment in Antarctica: global transportation and climate change8,40,41. For the former, both the volume and the pathways of ship traffic will increase over time as scientific research, commercial interests and tourism increase12. Certainly, measures can be put in place to reduce the probability of the transport of NIS propagules15. Whereas ballast water regulations have already been instituted under the Antarctic Treaty and International Maritime Organization, no international biosecurity measure for hull fouling (e.g. cf. Resolution MEPC.207(62)42) has entered into force yet in spite of strongly acknowledged risk for invasions in the Antarctic19. Nevertheless, the increased numbers of ships will inevitably lead to additional releases including in areas that are currently rarely visited. More worrisome, however, is climate change as the extreme conditions of Antarctic marine waters (water temperatures always <2 °C) represent a significant physiological barrier for species originating from more temperate regions. As temperatures in Southern Ocean increase43, more and more species will be able to establish and those that establish will have greater and greater impacts. Indeed, even small changes in water temperatures can have large effects44, and the most worrisome element of the information presented in this study is not that this particular population of mussels is poised to impact the benthic communities of Antarctica, but rather it provides a glimpse of “invasions future”: the vector has been identified, the pathways have been described and at least one of the environmental filters has failed. The ship traffic data have shed light on putative vectors and pathways of introduction and shown a strong potential of propagule and colonization pressures45 of Patagonian taxa. Moreover, Fildes Bay and other locations in the South Shetland Islands may become important stepping stones for further spread in Antarctica. Finally, completion of the larval period in such low temperatures represent a major step in the invasion process. Whether spawning occurred in situ from transported adults (e.g. on vessel hulls or in sea chests) or within their native range, followed by larval transport (e.g. within ballast tanks) cannot be ascertained12,14, but in any case, barriers may be weakening and the bar for invasion may be much lower than believed. The Antarctic constitutes a distinct biogeographic realm, and global warming will not only put charismatic native species at risk, but also lower dispersal and physiological barriers to NIS in intertidal and shallow waters18,27. Whether a result of direct anthropogenic causes or indirect biotic interactions, the resulting changes in the unique biotic assemblages of Antarctica will represent an irreversible change to one of the most unique marine biotas on the planet.
Source: Ecology - nature.com



