In this phase I/II single (only participant) blinded pilot trial, we tested safety and efficacy of A. soehngenii in metabolic syndrome subjects and found that daily ingestion of increasing A. soehngenii doses for 1 month was associated with increased fecal levels of A. soehngenii with greatest efficacy in the subjects who received the highest dose. The increase was transient (Supplementary Table 1 and Fig. 2), as 2 weeks after cessation most of the A. soehngenii was cleared from the feces. In line with our murine data14, we observed beneficial changes in bile acid metabolism, which combined with Anaerobutyricum spp. growth dynamics suggests that this bacterial strain survives passage through the gastrointestinal tract. Furthermore, we found that the relative abundance of administered A. soehngenii positively correlated with improved Rd (p = 0.044). Combined with the good safety profile, our data imply that the highest dose of the A. soehngenii is well tolerated and may be an additional treatment for insulin resistance.
This study takes the reductionist approach of reintroducing a bacterial therapeutic strain in metabolic syndrome subjects based on previous intervention trials5. We show that this approach is feasible, safe, and may induce a beneficial cardiovascular profile based on the introduced metabolically active bacterial strain. Interestingly, a recent paper showed that the increase in the levels of fecal propionate was causally linked to insulin resistance15, in line with our finding of decreased propionate upon A. soehngenii administration. However, both fecal and plasma SCFAs are notoriously difficult to measure due to volatility and assay detection limits16. Thus, the reduction in blood pressure in the highest dose group might be driven by SCFA-producing fecal bacterial strains17, despite not finding a significant effect on fecal SCFA levels. Finally, although the treatment efficacy of single-strain A. soehngenii was smaller than our findings on improved Rd upon lean donor FMT5,10, a recent FMT study from another group underscored these findings and showed that an enrichment of Anaerobutyricum spp. was associated with altered bile acids and clinical efficacy upon donor FMT in patients with ulcerative colitis18. In line, our results are similar to other human single-strain intervention studies demonstrating (in a subset of patients) an effect on insulin sensitivity after 12 weeks of supplementation with a high dose of Lactobacillus reuteri DSM 1793819. Upon the highest dose of A. soehngenii, half of metabolic syndrome subjects showed a significant improvement in glucose metabolism, paralleled by a concomitant increase in fecal levels of A. soehngenii after 4 weeks. This was corroborated by recent studies20,21 showing that engraftment of administered bacterial strains is only seen in a subset of treated subjects and depends on baseline fecal microbiota composition allowing engraftment and driving cohabitation between the endogenous microbiota and the exogenous bacterial strains21. We thus calculated that if baseline microbiota composition indeed drives the efficacy of engraftment of the A. soehngenii, we would need to treat about 20 metabolic syndrome subjects with this specific baseline microbiota composition with a dose of 1011 cells/day to be able to detect a significant increase in Rd. In contrast to our murine study14 and although fecal A. soehngenii increased significantly after 4 weeks of treatment, we observed no changes in fecal butyrate. While we cannot rule out that the effect of A. soehngenii administration is due to butyrate production16 from lactate and acetate in the small intestine6,7, we also observed that, upon A. soehngenii administration, plasma bile acid concentrations in the medium dose group changed with a predominant increase in plasma secondary bile acids, known to associate with improved glucose metabolism in insulin resistant subjects22. It has been previously observed in a human intervention trial using B. infantis that high concentrations (1010 CFU) of bacterial strains can induce a crowding effect resulting in less efficient dispersion of the bacteria in the intestine and thus in different clinical effects23. The fact that the medium group showed the most pronounced changes in bile acid composition may signify that it is the dose that best drives the endogenous-exogenous bacterial strain intestinal milieu for generation of secondary bile acids. Moreover, the reduction in fecal propionate levels in the same medium dose group, but not in other dose groups, aligns with this finding, although it may have been caused by the small number of subjects per treatment group. It has been increasingly recognized that intestinal microbiota play an important role in bile acid metabolism by synthesizing secondary bile acids from primary bile acids via deconjugation and dihydroxylation24. Next to their role in intestinal fat absorption, bile acids are crucial regulators of glucose and energy homeostasis24, and recent studies have shown that disturbances in bile acid metabolism may contribute to the pathogenesis of type 2 diabetes1,25,26. In line, our data in insulin resistant mice demonstrated that oral A. soehngenii treatment significantly changed levels of plasma bile acid14 and a previous probiotic human trial likewise showed altered plasma bile acids upon use of L. reuteri DSM 1793819.
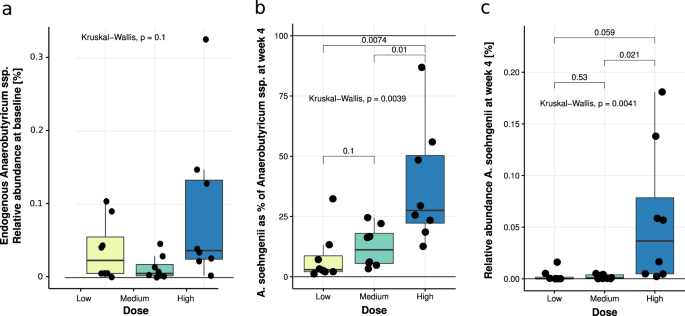
Interestingly, the estimated number of A. soehngenii cells present in the fecal sample after 4 weeks of A. soehngenii administration was orders of magnitude higher than the daily intake of A. soehngenii cells per day (Supplementary Fig. 1). The ratio was significantly larger for the low dose compared to the high-dose group, suggesting that low amounts of A. soehngenii cells may be better protected by the milk drink during gastrointestinal passage. Another, non-exclusive, possibility is that there is a strong competition for resources in the colon and that low amounts of A. soehngenii can compete better and multiply more than higher dosages. The higher replication signal of A. soehngenii in the drink (freshly frozen and stored after being grown in single-culture condition on optimized culture media) compared to that of Anaerobutyricum spp. in feces likely reflects lower growth rates of Anaerobutyricum spp. (including A. soehngenii) in the limited substrate and high competitive environment of the gut. A. soehngenii was grown in large scale production in a sucrose-based medium, found in a previous study to be protective during frozen storage27.
The genome of A. soehngenii was recently published and underlined significant differences compared to the (endogenous) A. hallii6. Altogether with the different SCFA production pattern and bacterial wall fatty acid membrane composition of A. soehngenii, and in line with our clinical findings, these data strongly suggest that A. soehngenii has specific properties. Nevertheless, the dose-dependent increase in fecal A. soehngenii levels upon treatment was not associated with major changes in gut microbiota diversity, consistent with the observations from our mice study14. However, we observed an inverse correlation between baseline abundance of P. copri and the change in Rd (rho = −0.41, p = 0.043). Also, a comparison of responders and non-responders at baseline found that responders had around 65% less P. copri when compared to non-responders (Supplementary Table 9). The relation between A. soehngenii and Prevotella copri might be of interest, as the latter strain has been linked to glucose metabolism in humans and may work synergistically with A. soehngenii on insulin-sensitizing effects28. Thus, future studies will have to focus on dissecting the therapeutic synergy of co-administrating other bacterial strains, together with A. soehngenii.
The rapid decrease in fecal A. soehngenii levels after 2 weeks cessation of daily administration (Fig. 2) occurred at the same time as the increase in plasma primary bile acids (Fig. 6). This is similar to findings in the study with L. reuteri19, suggesting that systemic effects may persist for several weeks after the administered strain’s concentration in feces falls. As expected, beneficial metabolic effects were not seen in all subjects in the highest dosage groups. It is likely that the administered A. soehngenii is either not maximally engrafting or is not active enough to induce these effects. Another study in infants showed that the administered strain did not engraft in all treated subjects29, although the baseline microbiota composition was not considered.
Our study has several limitations, including the nature of its study design, single-blinded for the participant only. Although not as powerful as a randomized clinical trial (RCT) in determining treatment effect, a single-blinded, dose-escalation study was chosen instead due to ethical considerations, as an important aim of this study was to determine whether a high daily dose of 1011 cells/day of A. soehngenii was safe and well tolerated in humans. During the trial, the viability of the 10 ml tubes that were stored at −80C was checked every 6 months. However, we did not determine the viability after home freezer storage during the 4 weeks intervention; we assumed viability loss, if any, to be similar in all households. The parameters used in the calculation of the ingested/secreted ratio of A. soehngenii may vary widely between as well as among individuals, thus the estimated ingested/secreted ratio values are approximations. Another limitation is the small group size and relatively short duration of treatment. When pooling subjects and looking at relative changes after 4 weeks of treatment, we observed a significant correlation between the relative abundance of administered A. soehngenii and the change in Rd. Thus, these outcomes could serve to guide power calculations for future intervention RCT trials with high-dosed bacterial strains such as A. soehngenii30. Moreover, as the goal of our study was to test safety and efficacy of different A. soehngenii dosages in humans, we did not compare different A. soehngenii strains, which will need to be done in future studies. The effect of the A. soehngenii on the phenotype of the participants may be mediated by unknown factors other than SCFA and secondary bile acids. Finally, stomach acid and oxygen affect viability of administered strains, which thus are independent of original ingested dose. However, the fact that the A. soehngenii strain showed the highest replication signal in the feces of subjects treated with the highest dose suggests that large daily amounts are needed. Future research will have to show whether protecting A. soehngenii against stomach acid and oxygen (e.g., by encapsulation and/or freeze-drying) will have greater therapeutic efficacy.
In conclusion, in this proof-of-concept pilot study, humans with metabolic syndrome were treated with a bacterial strain selected based on microbiota findings from our previous studies5,10,14. When all treatment groups were pooled, we observed a positive correlation between fecal A. soehngenii abundance and Rd. These results suggest that modulating the microbiota in humans may improve glucose metabolism and could therefore constitute a therapeutic modality in the treatment of type 2 diabetes. More research is needed on long-term effects and modes of delivery, which were beyond the scope of the current study. Nevertheless, we show here that using the current administration, high-dosed A. soehngenii is partially able to survive gastrointestinal tract passage and is accompanied by a beneficial safety profile. This provides a rationale for future A. soehngenii high-dose intervention trials in treatment-naive human subjects with metabolic syndrome.
Source: Ecology - nature.com


