Plant material and climate of the area where it was found
The examined plant material is from a very old, cultivated, domesticated type of rose found growing in the private garden of a house in the small village of Carballo (Concejo de Cangas del Narcea, in the Principality of Asturias, northern Spain), which nestles in the valley of the River Cibea. Several specimens of this type of rosebush may have been growing in this same garden before 1867 (and possibly before 1832) (personal communications from local inhabitants). Certainly, their long existence was known to several senior villagers. Their large roses with their many petals, notable colour and agreeable aroma, led to their being collected for use in the festival of Corpus Christi, the petals being thrown into the air as the religious procession passed by. Indeed, even if the flowering of the rose depends on temperature and humidity conditions, we could approximately predict flowering time thanks to the fact that the oral tradition had stablished that flowering occurred around the time of this festival (which falls on the ninth Sunday after the first spring full moon in the northern hemisphere). Today there are three such plants in the same garden; these are now being used to propagate more specimens.
Carballo lies in a mountainous area (with altitudes rising from 300 to 1700 m over just a few kilometres) that forms part of the Cantabrian Mountain Range, where there are many rivers with steep-sided valleys. Coastal humidity is held back by these mountains. Together these factors confer upon the area its particular microclimate, the details of which are provided in Supplementary Table S1 (data collected by an iMetos 2 agroclimate station present in Carballo since 2010).
Botanical characterisation and data of agronomic interest
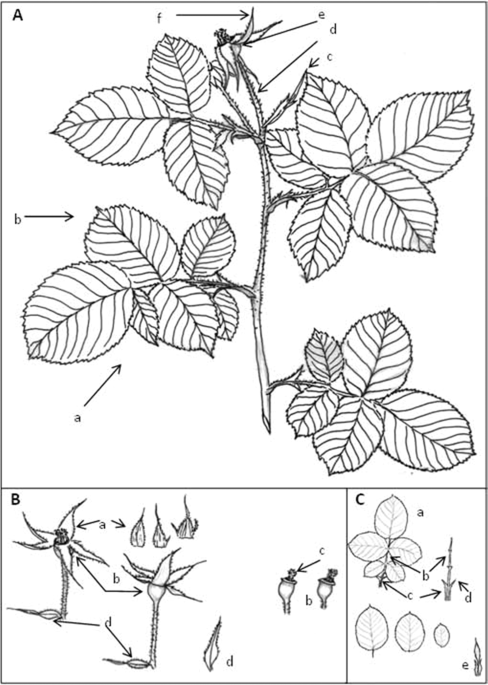
Plant characteristics were recorded over the growth cycle, collecting data on the features of the shoots, adult leaves, flowers and fruits. The flowers were inspected on 31st May 2018 (10 flowers collected from each of the three bushes). A botanical description was made following the protocol of the International Union for the Protection of New Varieties of Plants15 for the genus Rosa, as well as the descriptive method of Monserrat et al.10. Drawings and photographs of different plant organs were made. On the same 31st May, the number of flower buds, and the number of open roses per plant was recorded.
Between five and eight further roses were collected from each plant, and the number of petals per flower, the weight of the petals per flower and the weight of each petal recorded before freezing them at −30 °C until further analysis.
Molecular characterisation
DNA extraction
Fresh leaves were collected in April 2018 and stored at −80 °C for DNA extraction. Using a pestle and mortar, leaf material (0.2 g) was homogenised in 1 mL of CTAB buffer, as described by De la Rosa et al.28. DNA was then extracted using the Maxwell® PureFood Kit and Maxwell RSC Instrument (Promega Corporation, Madison, WI, USA) following the manufacture’s recommendations. The extracted DNA was resuspended in 100 µL of elution buffer (provided with the kit), and quantified using a Biodrop µLITE® spectrophotometer (BioDrop, Cambridge, UK). DNAs extracted from the roses ‘Belle de Crécy’, ‘Jolande d’Aragone’, and ‘Alain Blanchard’ (Scariot et al.17) were used as reference for further analyses.
STMS amplification
STMSs were amplified by PCR in a 20 µL reaction volume containing 2 µL 10X PCR buffer (100 mM Tris-HCL, pH 8.3, 500 mM KCl), 1.5 mM MgCl2, 0.5 µM of each primer, 200 µM dNTP, 0.5 U taq-DNA polymerase (i.e., AmpliTaq Gold DNA polymerase [Applied Biosystems, Foster City, CA, USA]) and 50 ng of template DNA. Amplifications were performed in a PTC 100 thermocycler (MJ Research, Watertown MA, USA). The primers used, developed by Esselink et al.29, were RhAB22, RhE2b, RhD221, RhO517, and RhP519. The forward primers were labelled with a fluorochome (6-FAM, HEX or NED). Amplification cycles consisted of an initial step of 11 min at 95 °C, followed by 26 cycles of 30 s at 95 °C, 40 s at 55 °C, 1 min 30 s at 72 °C, with a final extension step of 45 min at 72 °C.
Detection of STMS polymorphism
One microlitre of a mix containing amplification products of three differently labelled loci was added to 3 µL of a mix containing 10:2:1 parts formamide, GeneScan-350ROX size standard (Applied Biosystems) and a loading buffer (25 mM EDTA, 50 mg mL−1 blue dextran). Fluorescent samples were denatured at 95 °C for 5 min and the DNA fragments separated on a sequencing gel (4.25% acrylamide, 1X TBE buffer, 6 M urea) using an ABI-PRISM 377 DNA sequencer running GeneScan software (Applied Biosystems).
Data analysis
The obtained STMS peaks were scored as discrete variables (namely allelic phenotype), using 1 or 0 to indicate the presence or the absence of each fragment. Data were compared with the original dataset reported by Scariot et al.17, made available by the authors. The genetic distance between pairs of accessions was estimated on the basis of the Nei coefficient and a principal coordinate analysis (PCA) was conducted using GeneAlEx 6.330.
Histological characterisation of the petals
Several petals from each of the three rosebushes were thawed to room temperature and fixed in FAA (90% 70° ethanol, 5% glacial acetic acid, 5% formaldehyde) for 48 h. They were then transferred to 70° alcohol, passed through a dehydrating series of ethanol solutions (using isoamyl acetate as an intermediary liquid) and set in paraplast blocks. These were cut using a 12 µm microtome and deposited on microscope slides. Some preparations were stained with safranin and fast green (before being immersed in xylene to remove the paraffin), mounted in Entellan, and examined using a Nikon E600 microscope (bright field, polarized light and epifluorescence microscopy).
For histochemical detection of lipids, small fragments of petals were fixed for 24 h in 4% paraformaldehyde in 0.1 M phosphate buffer (PB). The samples were rinsed with the same buffer and cryoprotected by passing through sucrose solutions in increasing concentration (10, 20, and 30%). Sections (12 µm thick) were obtained in a cryostat and collected on gelatin-coated slides. The stain was performed with the Sudan III technique following the standard method described in Kiernan31. The sections were examined with an Olympus BX51 microscope and photographed with an Olympus DP71 digital camera.
Fragments of the fixed petals were also passed through a dehydrating series of ethanol solutions and after critical point drying were gold-covered and examined using a FEI Quanta 600 environmental scanning electron microscope (ESEM).
Analysis of petal volatile compounds
Six to eight more flowers from each of the three rosebushes were collected, their petals separated, weighed and stored separately (by bush) at −80 °C. A portion of those from each bush—Bush 1 = 30.46 g; Bush 2 = 38.33 g; Bush 3 = 39.90 g—was lyophilised using a Gamma 2–16 LSCplus lyophilising device (CHRIST, Osterode am Harz, Germany) at 0.10 mbars (lyophilised Bush 1 = 3.4 g; lyophilised Bush 2 = 4.67 g; lyophilised Bush 3 = 3.29 g) and sent for volatile compound analysis at the Instituto de Ciencia y Tecnología de los Alimentos y Nutrición–CSIC (Madrid, Spain).
The volatile compound profile of the essential oil of a Damask rose of French origin (Rose absolute, Moroccan, Ref.W298816-Sample-K) was examined as a reference against which to compare the studied petals. Samples for analysis were prepared in duplicate on the same day and maintained refrigerated until use. Samples of 0.05 g of each petal sample, or 20 µl of the Damask oil, were placed in a 20 mL glass headspace vial with a silicon/PTFE septum screw cap. Analyses were undertaken over two days, using one of the replicates each day, employing divinylbenzene/carboxen fibres (Ref. 57328-U Supelco [Merck KGaA, Darmstadt, Germany]). The volatile compounds of each were extracted by solid phase microextraction (SPME) and analysed by GC-MS (CG: Agilent 6890N device [Agilent Technologies, Santa Clara, CA, USA]; MS: 5973 device [Agilent Technologies, Santa Clara, CA, USA]) running MSD Chemstation software.
The technical details for CG-MS included equilibration of the heating plate at 50 °C for 30 min and 50 °C for 15 min extraction with the fibe; desorption of the injector at 240 °C for 15 min; volatile compounds column DB-WAXetr (polyethylene glycol 60 m, 320 × 0.25 μm); carrying gas – helium, constant flow (1.3 mL/min); Injection – desorption SPME splitless, 240 °C; heating gradient −40 °C 4 min, 4 °C/min 110 °C, 6 °C/min 180 °C, 8 °C/min 240 °C 15 min; auxiliary temperature 250 °C, detector temperature 230 °C; mass range (m/z)—27–450; data treatment: MassHunter Qualitative Analysis B.07.00 software.
Compounds were identified by comparison against the spectra in the Wiley Registry 7th Edition Spectral Library and the National Institute of Standards and Technology 2008 Mass Spectral Library (NIST 08), and by calculating linear retention indices with respect to a series of alkanes (C6-C20).
Source: Ecology - nature.com


