Kallmeyer, J., Pockalny, R., Adhikari, R. R., Smith, D. C. & D’Hondt, S. Global distribution of microbial abundance and biomass in subseafloor sediment. Proceedings of the National Academy of Sciences of the United States of America 109, 16213–16216, https://doi.org/10.1073/pnas.1203849109 (2012).
Jørgensen, B. B. & Marshall, I. P. G. In Annual Review of Marine Science, Vol 8 Vol. 8 Annual Review of Marine Science (eds. Carlson, C. A. & Giovannoni, S. J.) 311–332 (2016).
Braun, S. et al. Microbial turnover times in the deep seabed studied by amino acid racemization modelling. Scientific Reports 7, 5680, https://doi.org/10.1038/s41598-017-05972-z (2017).
Parkes, R. J., Cragg, B. A. & Wellsbury, P. Recent studies on bacterial populations and processes in subseafloor sediments: A review. Hydrogeology Journal 8, 11–28, https://doi.org/10.1007/pl00010971 (2000).
Contreras, S. et al. Cyclic 100-ka (glacial-interglacial) migration of subseafloor redox zonation on the Peruvian shelf. Proceedings of the National Academy of Sciences 110, 18098–18103 (2013).
Parkes, R. J. et al. Deep sub-seafloor prokaryotes stimulated at interfaces over geological time. Nature 436, 390–394, https://doi.org/10.1038/nature03796 (2005).
Morono, Y. et al. Carbon and nitrogen assimilation in deep subseafloor microbial cells. Proceedings of the National Academy of Sciences of the United States of America 108, 18295–18300, https://doi.org/10.1073/pnas.1107763108 (2011).
Trembath-Reichert, E. et al. Methyl-compound use and slow growth characterize microbial life in 2-km-deep subseafloor coal and shale beds. Proceedings of the National Academy of Sciences of the United States of America 114, E9206–E9215, https://doi.org/10.1073/pnas.1707525114 (2017).
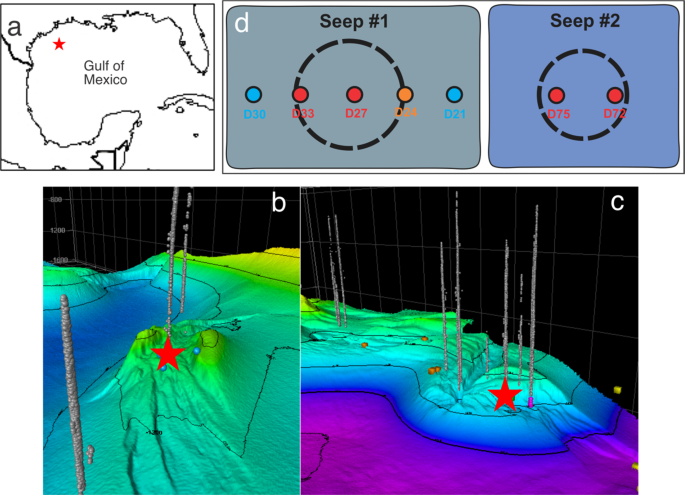
Garcia-Pineda, O. et al. Transience and persistence of natural hydrocarbon seepage in Mississippi Canyon, Gulf of Mexico. Deep-Sea Research Part Ii-Topical Studies in Oceanography 129, 119–129, https://doi.org/10.1016/j.dsr2.2015.05.011 (2016).
Chakraborty, A. et al. Thermophilic endospores associated with migrated thermogenic hydrocarbons in deep Gulf of Mexico marine sediments. ISME Journal 12, 1895–1906, https://doi.org/10.1038/s41396-018-0108-y (2018).
Lloyd, K. G. et al. Spatial structure and activity of sedimentary microbial communities underlying a Beggiatoa spp. mat in a Gulf of Mexico hydrocarbon seep. PLoS One 5, e8738, https://doi.org/10.1371/journal.pone.0008738 (2010).
Dekas, A. E. et al. Widespread nitrogen fixation in sediments from diverse deep-sea sites of elevated carbon loading. Environmental microbiology 20, 4281–4296 (2018).
Dong, X. et al. Metabolic potential of uncultured bacteria and archaea associated with petroleum seepage in deep-sea sediments. Nature communications 10, 1816 (2019).
Scott, N. M. et al. The microbial nitrogen cycling potential is impacted by polyaromatic hydrocarbon pollution of marine sediments. Frontiers in Microbiology 5, 108, https://doi.org/10.3389/fmicb.2014.00108 (2014).
Mason, O. U. et al. Metagenomics reveals sediment microbial community response to Deepwater Horizon oil spill. ISME Journal 8, 1464–1475, https://doi.org/10.1038/ismej.2013.254 (2014).
Dombrowski, N., Seitz, K. W., Teske, A. P. & Baker, B. J. Genomic insights into potential interdependencies in microbial hydrocarbon and nutrient cycling in hydrothermal sediments. Microbiome 5, 106, https://doi.org/10.1186/s40168-017-0322-2 (2017).
Dombrowski, N., Teske, A. P. & Baker, B. J. Expansive microbial metabolic versatility and biodiversity in dynamic Guaymas Basin hydrothermal sediments. Nature. Communications 9, 4999 (2018).
Yoshimura, K. M., York, J. & Biddle, J. F. Impacts of salinity and oxygen on particle-associated microbial communities in the Broadkill River, Lewes DE. Frontiers in Marine Science 5, 100, https://doi.org/10.3389/fmars.2018.00100 (2018).
Zhao, R., Hannisdal, B., Mogollon, J. M. & Jørgensen, S. L. Nitrifier abundance and diversity peak at deep redox transition zones. Scientific Reports 9, 8633 (2019).
Denman, S. E., Tomkins, N. & McSweeney, C. S. Quantitation and diversity analysis of ruminal methanogenic populations in response to the antimethanogenic compound bromochloromethane. FEMS Microbiol. Ecol. 62, 313–322, https://doi.org/10.1111/j.1574-6941.2007.00394.x (2007).
Andrews, S. FastQC: a quality control tool for high throughput sequence data. https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (2010).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120, https://doi.org/10.1093/bioinformatics/btu170 (2014).
Gruber-Vodicka, H. R., Seah, B. K. & Pruesse, E. phyloFlash — Rapid SSU rRNA profiling and targeted assembly from metagenomes. bioRxiv, 521922, https://doi.org/10.1101/521922 (2019).
Boyd, J. A., Woodcroft, B. J. & Tyson, G. W. GraftM: a tool for scalable, phylogenetically informed classification of genes within metagenomes. Nucleic Acids Research 46, e59, https://doi.org/10.1093/nar/gky174 (2018).
Bushnell, B. BBMap: a fast, accurate, splice-aware aligner. (Ernest Orlando Lawrence Berkeley National Laboratory, Berkeley, CA (US), 2014).
Rognes, T., Flouri, T., Nichols, B., Quince, C. & Mahe, F. VSEARCH: a versatile open source tool for metagenomics. PeerJ 4, e2584, https://doi.org/10.7717/peerj.2584 (2016).
Quast, C. et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Research 41, D590–D596, https://doi.org/10.1093/nar/gks1219 (2013).
Cole, J. R. et al. The Ribosomal Database Project (RDP-II): sequences and tools for high-throughput rRNA analysis. Nucleic acids research 33, D294–D296 (2005).
Li, D. H., Liu, C. M., Luo, R. B., Sadakane, K. & Lam, T. W. MEGAHIT: an ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics 31, 1674–1676, https://doi.org/10.1093/bioinformatics/btv033 (2015).
Wu, Y. W., Simmons, B. A. & Singer, S. W. MaxBin 2.0: an automated binning algorithm to recover genomes from multiple metagenomic datasets. Bioinformatics 32, 605–607, https://doi.org/10.1093/bioinformatics/btv638 (2016).
Parks, D. H., Imelfort, M., Skennerton, C. T., Hugenholtz, P. & Tyson, G. W. CheckM: assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Research 25, 1043–1055, https://doi.org/10.1101/gr.186072.114 (2015).
Seah, B. K. & Gruber-Vodicka, H. R. gbtools: interactive visualization of metagenome bins in R. Frontiers in Microbiology 6, 1451 (2015).
R Development Core Team. R: A language and environment for statistical computing. R foundation for statistical computing Vienna, Austria (2011).
Wickham, H. ggplot2: elegant graphics for data analysis. (Springer, 2016).
Seemann, T. Prokka: rapid prokaryotic genome annotation. Bioinformatics 30, 2068–2069, https://doi.org/10.1093/bioinformatics/btu153 (2014).
Hyatt, D. et al. Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinformatics 11, 119, https://doi.org/10.1186/1471-2105-11-119 (2010).
Altschul, S. F. et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Research 25, 3389–3402, https://doi.org/10.1093/nar/25.17.3389 (1997).
Kanehisa, M., Sato, Y. & Morishima, K. BlastKOALA and GhostKOALA: KEGG Tools for Functional Characterization of Genome and Metagenome Sequences. Journal of Molecular Biology 428, 726–731, https://doi.org/10.1016/j.jmb.2015.11.006 (2016).
Huerta-Cepas, J. et al. eggNOG 4.5: a hierarchical orthology framework with improved functional annotations for eukaryotic, prokaryotic and viral sequences. Nucleic Acids Research 44, D286–D293, https://doi.org/10.1093/nar/gkv1248 (2016).
Graham, E., Heidelberg, J. & Tully, B. Potential for primary productivity in a globally-distributed bacterial phototroph. The ISME journal 12, 1861–1866 (2018).
Sorek, R. et al. Genome-wide experimental determination of barriers to horizontal gene transfer. Science 318, 1449–1452, https://doi.org/10.1126/science.1147112 (2007).
Hug, L. A. et al. A new view of the tree of life. Nature microbiology 1, 16048 (2016).
Altschul, S. F., Gish, W., Miller, W., Myers, E. W. & Lipman, D. J. Basic local alignment search tool. Journal of Molecular Biology 215, 403–410, https://doi.org/10.1006/jmbi.1990.9999 (1990).
Campbell, J. H. et al. UGA is an additional glycine codon in uncultured SR1 bacteria from the human microbiota. Proceedings of the National Academy of Sciences of the United States of America 110, 5540–5545, https://doi.org/10.1073/pnas.1303090110 (2013).
Eren, A. M. et al. Anvi’o: an advanced analysis and visualization platformfor ‘omics data. PeerJ 3, e1319, https://doi.org/10.7717/peerj.1319 (2015).
Edgar, R. C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research 32, 1792–1797, https://doi.org/10.1093/nar/gkh340 (2004).
Capella-Gutierrez, S., Silla-Martinez, J. M. & Gabaldon, T. trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 25, 1972–1973, https://doi.org/10.1093/bioinformatics/btp348 (2009).
Stamatakis, A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30, 1312–1313, https://doi.org/10.1093/bioinformatics/btu033 (2014).
Letunic, I. & Bork, P. Interactive tree of life (iTOL) v3: an online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Research 44, W242–W245, https://doi.org/10.1093/nar/gkw290 (2016).
Katoh, K. & Standley, D. M. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution 30, 772–780, https://doi.org/10.1093/molbev/mst010 (2013).
Nguyen, L. T., Schmidt, H. A., von Haeseler, A. & Minh, B. Q. IQ-TREE: A Fast and Effective Stochastic Algorithm for Estimating Maximum-Likelihood Phylogenies. Molecular Biology and Evolution 32, 268–274, https://doi.org/10.1093/molbev/msu300 (2015).
Kalyaanamoorthy, S., Minh, B. Q., Wong, T. K. F., von Haeseler, A. & Jermiin, L. S. ModelFinder: fast model selection for accurate phylogenetic estimates. Nature Methods 14, 587, https://doi.org/10.1038/nmeth.4285 (2017).
Lau, E., Fisher, M. C., Steudler, P. A. & Cavanaugh, C. M. The methanol dehydrogenase gene, mxaF, as a functional and phylogenetic marker for Proteobacterial methanotrophs in natural environments. PLoS One 8, e56993, https://doi.org/10.1371/journal.pone.0056993 (2013).
Lazar, C. S., Baker, B. J., Seitz, K. W. & Teske, A. P. Genomic reconstruction of multiple lineages of uncultured benthic archaea suggests distinct biogeochemical roles and ecological niches. ISME Journal 11, 1058–1058, https://doi.org/10.1038/ismej.2017.8 (2017).
Hug, L. A. et al. Critical biogeochemical functions in the subsurface are associated with bacteria from new phyla and little studied lineages. Environmental Microbiology 18, 159–173, https://doi.org/10.1111/1462-2920.12930 (2016).
Albertsen, M. et al. Genome sequences of rare, uncultured bacteria obtained by differential coverage binning of multiple metagenomes. Nature Biotechnology 31, 533–538, https://doi.org/10.1038/nbt.2579 (2013).
Parks, D. H. et al. Recovery of nearly 8,000 metagenome-assembled genomes substantially expands the tree of life. Nature Microbiology 2, 1533–1542, https://doi.org/10.1038/s41564-017-0012-7 (2017).
Sczyrba, A. et al. Critical sssessment of metagenome interpretation-a benchmark of metagenomics software. Nature Methods 14, 1063–1071, https://doi.org/10.1038/nmeth.4458 (2017).
Garcia-Pineda, O. et al. Transience and persistence of natural hydrocarbon seepage in Mississippi Canyon, Gulf of Mexico. Deep Sea Research Part II: Topical Studies in Oceanography 129, 119–129 (2016).
Roller, B. R. K., Stoddard, S. F. & Schmidt, T. M. Exploiting rRNA operon copy number to investigate bacterial reproductive strategies. Nature. Microbiology 1, 16160, https://doi.org/10.1038/nmicrobiol.2016.160 (2016).
Stoddard, S. F., Smith, B. J., Hein, R., Roller, B. R. K. & Schmidt, T. M. rrnDB: improved tools for interpreting rRNA gene abundance in bacteria and archaea and a new foundation for future development. Nucleic Acids Research 43, D593–D598, https://doi.org/10.1093/nar/gku1201 (2015).
LaRowe, D. E. & Amend, J. P. Catabolic rates, population sizes and doubling/replacement times of microorganisms in natural settings. American Journal of Science 315, 167–203 (2015).
Lomstein, B. A., Langerhuus, A. T., D’Hondt, S., Jorgensen, B. B. & Spivack, A. J. Endospore abundance, microbial growth and necromass turnover in deep sub-seafloor sediment. Nature 484, 101–104, https://doi.org/10.1038/nature10905 (2012).
Vuillemin, A. et al. Archaea dominate oxic subseafloor communities over multimillion-year time scales. Science Advances 5, eaaw4108, https://doi.org/10.1126/sciadv.aaw4108 (2019).
Coskun, Ö. K., Özen, V., Wankel, S. D. & Orsi, W. D. Quantifying population-specific growth in benthic bacterial communities under low oxygen using H218O. The ISME Journal 13, 1546–1559, https://doi.org/10.1038/s41396-019-0373-4 (2019).
Eloe-Fadrosh, E. A., Ivanova, N. N., Woyke, T. & Kyrpides, N. C. Metagenomics uncovers gaps in amplicon-based detection of microbial diversity. Nature Microbiology 1, 15032 (2016).
Fischer, M. A., Güllert, S., Neulinger, S. C., Streit, W. R. & Schmitz, R. A. Evaluation of 16S rRNA Gene Primer Pairs for Monitoring Microbial Community Structures Showed High Reproducibility within and Low Comparability between Datasets Generated with Multiple Archaeal and Bacterial Primer Pairs. Frontiers in Microbiology 7, 1297, https://doi.org/10.3389/fmicb.2016.01297 (2016).
Tully, B. J. & Heidelberg, J. F. Potential mechanisms for microbial energy acquisition in oxic deep-sea sediments. Appl. Environ. Microbiol. 82, 4232–4243, https://doi.org/10.1128/aem.01023-16 (2016).
Dekas, A. E., Poretsky, R. S. & Orphan, V. J. Deep-sea Archaea fix and share nitrogen in methane-consuming microbial consortia. Science 326, 422–426, https://doi.org/10.1126/science.1178223 (2009).
Dekas, A. E., Chadwick, G. L., Bowles, M. W., Joye, S. B. & Orphan, V. J. Spatial distribution of nitrogen fixation in methane seep sediment and the role of the ANME archaea. Environmental Microbiology 16, 3012–3029, https://doi.org/10.1111/1462-2920.12247 (2014).
Joye, S. B. et al. The anaerobic oxidation of methane and sulfate reduction in sediments from Gulf of Mexico cold seeps. Chemical Geology 205, 219–238, https://doi.org/10.1016/j.chemgeo.2003.12.019 (2004).
Barco, R. A. et al. New insight into microbial iron oxidation as revealed by the proteomic profile of an obligate iron-oxidizing chemolithoautotroph. Appl. Environ. Microbiol. 81, 5927–5937, https://doi.org/10.1128/aem.01374-15 (2015).
Dyksma, S., Pjevac, P., Ovanesov, K. & Mussmann, M. Evidence for H2 consumption by uncultured Desulfobacterales in coastal sediments. Environmental microbiology 20, 450–461 (2018).
Castelle, C. J. et al. Extraordinary phylogenetic diversity and metabolic versatility in aquifer sediment. Nature Communications 4, 2120, https://doi.org/10.1038/ncomms3120 (2013).
Meng, J. et al. Genetic and functional properties of uncultivated MCG archaea assessed by metagenome and gene expression analyses. The ISME journal 8, 650–659 (2014).
Dahle, H., Okland, I., Thorseth, I. H., Pederesen, R. B. & Steen, I. H. Energy landscapes shape microbial communities in hydrothermal systems on the Arctic Mid-Ocean Ridge. ISME Journal 9, 1593–1606, https://doi.org/10.1038/ismej.2014.247 (2015).
Mußmann, M., Pjevac, P., Kruger, K. & Dyksma, S. Genomic repertoire of the Woeseiaceae/JTB255, cosmopolitan and abundant core members of microbial communities in marine sediments. ISME Journal 11, 1276–1281, https://doi.org/10.1038/ismej.2016.185 (2017).
Ettwig, K. F. et al. Nitrite-driven anaerobic methane oxidation by oxygenic bacteria. Nature 464, 543–548, https://doi.org/10.1038/nature08883 (2010).
van de Vossenberg, J. et al. The metagenome of the marine anammox bacterium ‘Candidatus Scalindua profunda’ illustrates the versatility of this globally important nitrogen cycle bacterium. Environmental Microbiology 15, 1275–1289, https://doi.org/10.1111/j.1462-2920.2012.02774.x (2013).
Craine, J. M., Morrow, C. & Fierer, N. Microbial nitrogen limitation increases decomposition. Ecology 88, 2105–2113, https://doi.org/10.1890/06-1847.1 (2007).
Musat, F., Harder, J. & Widdel, F. Study of nitrogen fixation in microbial communities of oil-contaminated marine sediment microcosms. Environmental Microbiology 8, 1834–1843, https://doi.org/10.1111/j.1462-2920.2006.01069.x (2006).
Source: Ecology - nature.com


