Marine bivalves such as oysters and scallops secrete calcified shells as a supporting frame for their soft bodies and for protection from predators1,2, which is thought to be one of the key factors that trigger the expansion of bivalves at the dawn of the Cambrian times16. The early shell formation of bivalve larvae requires sufficient endogenous energy sources such as glucose, lipids and protein. In recent years, larval shell formation of marine bivalves has been severely affected by OA, resulting in vast mortality worldwide. Since previous study demonstrated that the level of reserves was only slightly higher than that required for shell formation in bivalve larvae10, it was hypothesized that OA might influence larval shell formation by disrupting the energy metabolic process. Although some progress has been made on revealing the metabolic bases of bivalve larvae under OA threat, much work is still needed to illustrate the precise metabolic pathways of lipids, carbohydrates and proteins during the formation of initial shell (PDI shell). In the present study, metabolomic and transcriptomic approaches were employed to study the metabolic variations in oyster larvae during formation of calcified shell and upon experimental OA, hoping to identify metabolites related to lipid/carbohydrate/protein metabolism during PDI shell formation and to reveal the negative effects of OA on oyster larvae from a point of view of energy metabolism.
Oyster larvae at three developmental stages (15 hpf (“early”), 17 hpf (“middle”) and 21 hpf (“late”)) were collected for metabolomic analysis since they were the key stages for the formation of calcified shells in oyster larvae. Totally 230 chemical compounds were identified from the present dataset, most of which were highly expressed in the “middle” stage. Overexpression of these metabolites indicated that a high level of energy metabolism was demanded for the formation of calcified shell. Using Random Forest and the Biochemical Importance Plot methods, a vast array of differentially expressed compounds related to energy metabolism at the “middle” stage were identified, such as glucose, glutarylcarnitine (C5), β-hydroxyisovaleroylcarnitine, 3-methylglutarylcarnitine (C6) and acetylcarnitine (C2) and ribose (Fig. 2). β -hydroxyisovalerylcarnitine, 3-methylglutarylcarnitine, glutarylcarnitine and acetylcarnitine are key intermediates of protein phosphorylation and amino acid oxidation17, while putrescine, 5-methylthioadenosine (MTA) and spermine were involved in the urea cycle18. Amino acids, derived largely from protein in the diet or from degradation of intracellular proteins, are the final class of biomolecules whose oxidation makes a significant contribution to the generation of metabolic energy19. Meanwhile, amino acid catabolism results in waste ammonia, which needs a way to be excreted since they are toxic20. As for most aquatic organisms, they excrete ammonia by diluting it by water outside the organism or converting it into a less toxic substance such as urea or uric21. In the present study, metabolites of both amino acid oxidation pathway and urea cycle pathway were overexpressed at the early D-shape larvae (“middle”) stage, which was key period for the PDI shell formation since a calcified shell was formed to cover the chitin one2. Besides, experimental OA was able to significantly suppress the mRNA expression of genes including DNA-directed RNA polymerase II submit spb 7, sparc-related modular calcium-binding protein 1-like, calcium-dependent protein kinase 31, ATP synthase lipid-binding mitochondrial-like, and ATP synthase subunit mitochondrial-like, which were related to protein metabolism and ATP synthesis22. Besides, research in marine bivalves proved that the interaction of seawater acidification and elevated temperature led to further expression of amino acid metabolism23. Therefore, results in the present study suggested that protein metabolism by amino acid oxidation could be a critical source of endogenous energy for the formation of initial shell in oyster larvae. OA inhibited larval shell formation by suppressing amino acid metabolism and resulted in a lack of ATP synthesis, which might then cause a failure of delay in PDI shell formation.
Apart from amino acid oxidation, glycolysis and pentose phosphate pathway were also found to be significant energy sources for initial shell formation in oyster larvae11,24. Glucose and ribose were highly expressed in the “middle” stage. Glucose is the most important source of energy in all organisms. In the present study, metabolites related to glucose metabolism through glycolysis and pentose phosphate pathway were identified during initial shell formation, and transcriptomic analysis illustrated that the expression genes related to glycolysis, such as pyruvate kinase, hexokinase and aldolase, was obviously inhibited upon experimental OA. These results were inconsistent with previous reports that OA could up-regulate energy metabolism in both adult and larval marine bivalves. For example, it was said that when oyster C. gigas received an acute OA treatment, the alanine and ATP levels in mantle tissue decreased significantly whereas an increase in succinate levels was observed in gill tissue25. Thus, results from the present and previous studies suggested that glucose metabolism through glycolysis and pentose phosphate pathway should be another crucial source of energy supply for initial shell formation in oyster, which could be severely affected by OA and resulted in a failure or delay in larval shell formation.
Furthermore, fatty acids metabolism was found to be the third energy source for PDI shell formation in oyster larvae. On one hand, metabolites related to fatty acids metabolism including succinylcarnitine, acetylcanitine, flavin mononucleotide (FMN), myristate (14:0), myristoleate (14:1n5), palmitoleate (16:1n7) and stearidonate (18:4n3) were highly expressed in the “middle” stage. On the other hand, several GO terms related to fatty acids related were also identified from the transcriptomic data, including phospholipid metabolic process, fatty acid metabolic process, and cellular lipid metabolic process (Fig. 5). In addition, experimental OA could suppress mRNA expression of lambda-crystallin homolog, tyrosine hydroxylase and acyl-coenzyme a thioesterase mitochondrial, which were key molecules for fatty acid metabolism26. Fatty acids yield the most ATP on an energy per gram basis, when they are completely oxidized to CO2 and water by beta oxidation and the citric acid cycle27. The metabolites identified in the present study were responsible for the activation of fatty acid degradation and could induce an increase of ATP synthesis. However, the metabolism of lipids could be negatively regulated by OA. In the pearl oyster P. fucata, genes associated with the “fatty acid biosynthesis” pathways were significantly enriched under acidification treatment13. And, unigenes involved in “fatty acid metabolism”, but not “glycerol metabolism”, are differentially expressed upon pH 7.8 treatment in P. fucata28. Like proteins and glucose, balanced lipid metabolism was also critical for PDI shell formation in oyster larvae24. And, results in the present study indicated that OA might influence initial shell formation of oyster larvae by inhibiting the processes of fatty acid metabolism. Furthermore, activities related to “fatty acid metabolic process” was slightly inhibited upon moderate acidification treatment (pH 7.7) and severely inhibited upon severe acidification treatment (pH 7.4) comparing with normal group, suggesting that moderate acidification treatment could barely influence fatty acid metabolism, while such process could be dramatically suppressed under severe acidification treatment.
The above results evidenced that formation of the initial shell in oyster larvae required endogenous energy supplied by amino acid oxidation, glycolysis, pentose phosphate pathway and fatty acid metabolism. These metabolic activities were severely affected by experimental OA, resulting in a failure or delay in PDI shell formation. In order to reveal the link between energy metabolism and shell formation, the expression patterns of shell formation-related genes were further investigated. By analyzing the transcriptomic data of oyster larvae under experimental OA, expressions of genes related to key processes in larvae shell formation were significantly inhibited, which included calcium transportation, bicarbonate transport, and organic matrix. These results suggested that the mobilization of calcium in oyster larvae was significantly disrupted upon experimental OA treatment. According to our previous study, oyster carbonic anhydrases (CA) was able to modulate intracellular pH (pHi) of oyster haemocytes under CO2 exposure29. Meanwhile, it was found that elevated CO2 caused the decrease of intracellular Ca2+ in haemocytes. The inhibition of CA by acetazolamide and suppression of CgCA gene via RNA interference increased the intracellular Ca2+ in haemocytes30. The above results suggested that initial shell formation in oyster larvae required endogenous energy supplied by amino acid oxidation, glycolysis, pentose phosphate pathway and fatty acid metabolism. Experimental OA affected such process by disrupting the balanced energy metabolism, which might alter the allocation of metabolic energy and further suppress the mobilization of calcium in oyster larvae. Moreover, activities related to “organic matrix” and “glycolysis” was activated upon moderate acidification treatment (pH 7.7) and inhibited upon severe acidification treatment (pH 7.4), which indicated that moderate acidification treatment could activate the stress response in oyster to sustain homeostasis upon acidifying environment, while the severe acidification treatment would significantly suppress normal physiological activities.
In conclusion, metabolomic and transcriptomic approaches were employed to investigate the energy metabolism of oyster larvae during PDI shell formation and under experimental OA treatment (Fig. 7). Results in the present study suggested that formation of the initial shell required endogenous energy coming from amino acid oxidation, glycolysis, pentose phosphate pathway and fatty acid metabolism. These metabolic activities could be severely inhibited by experimental OA, which might alter the allocation of metabolic energy. Insufficient endogenous energy supply then suppressed the mobilization of calcium and resulted in a failure or delay in PDI shell formation.
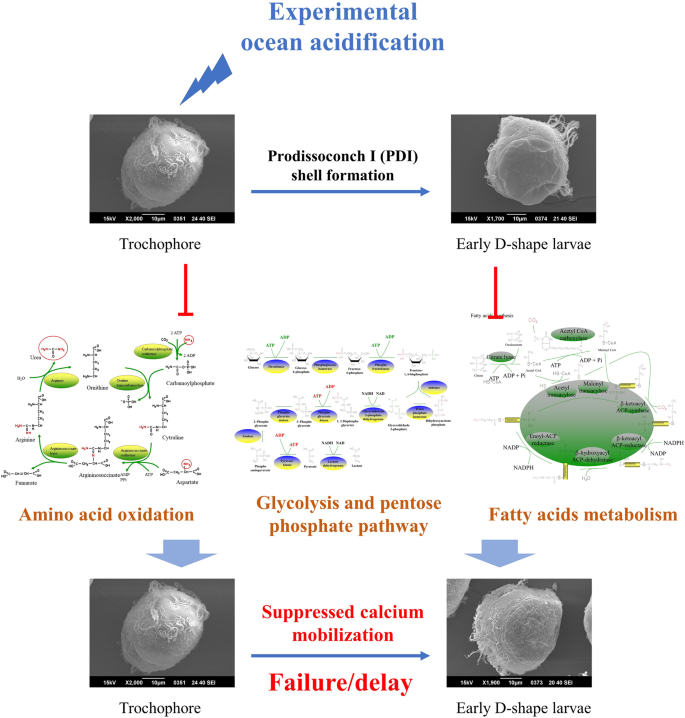
Schema illustrating how OA affected initial shell formation in oyster larvae. The formation of the initial shell in oyster larvae required endogenous energy coming from amino acid oxidation, glycolysis, pentose phosphate pathway and fatty acid metabolism. These metabolic activities could be severely inhibited by experimental OA, which might alter the allocation of metabolic energy. Insufficient endogenous energy supply then suppressed the mobilization of calcium and resulted in a failure or delay in PDI shell formation.
Source: Ecology - nature.com



