Pfeffer, C. et al. Filamentous bacteria transport electrons over centimetre distances. Nature 491, 218 (2012).
Nielsen, L. P., Risgaard-Petersen, N., Fossing, H., Christensen, P. B. & Sayama, M. Electric currents couple spatially separated biogeochemical processes in marine sediment. Nature 463, 1071–1074 (2010).
Marzocchi, U. et al. Electric coupling between distant nitrate reduction and sulfide oxidation in marine sediment. ISME J. 8, 1682 (2014).
Burdorf, L. D. W. et al. Long-distance electron transport occurs globally in marine sediments. Biogeosciences 14, 683–701 (2017).
Malkin, S. Y. et al. Natural occurrence of microbial sulphur oxidation by long-range electron transport in the seafloor. ISME J. 8, 1843–1854 (2014).
Risgaard-Petersen, N. et al. Cable bacteria in freshwater sediments. Appl. Environ. Microbiol. 81, 6003–6011 (2015).
Müller, H. et al. Long-distance electron transfer by cable bacteria in aquifer sediments. ISME J. 10, 2010–2019 (2016).
Müller, H., Marozava, S., Probst, A. J. & Meckenstock, R. U. Groundwater cable bacteria conserve energy by sulfur disproportionation. ISME J. 14, 623–634 (2020).
Kjeldsen, K. U. et al. On the evolution and physiology of cable bacteria. Proc. Natl. Acad. Sci. USA 116, 19116–19125 (2019).
Risgaard-Petersen, N., Revil, A., Meister, P. & Nielsen, L. P. Sulfur, iron-, and calcium cycling associated with natural electric currents running through marine sediment. Geochim. Cosmochim. Acta 92, 1–13 (2012).
Rao, A. M., Malkin, S. Y., Hidalgo-Martinez, S. & Meysman, F. J. The impact of electrogenic sulfide oxidation on elemental cycling and solute fluxes in coastal sediment. Geochim. Cosmochim. Acta 172, 265–286 (2016).
Sandfeld, T., Marzocchi, U., Petro, C., Schramm, A. & Risgaard-Petersen, N. Electrogenic sulfide oxidation mediated by cable bacteria stimulates sulfate reduction in freshwater sediments. ISME J. https://doi.org/10.1038/s41396-020-0607-5 (2020).
van der Gon, H. A. D., van Bodegom, P. M., Wassmann, R., Lantin, R. S. & Metra-Corton, T. M. Sulfate-containing amendments to reduce methane emissions from rice fields: mechanisms, effectiveness and costs. Mitig. Adapt. Strat. Glob. Change 6, 71–89 (2001).
Kristjansson, J. K., Schönheit, P. & Thauer, R. K. Different Ks values for hydrogen of methanogenic bacteria and sulfate reducing bacteria: an explanation for the apparent inhibition of methanogenesis by sulfate. Arch. Microbiol. 131, 278–282 (1982).
Schönheit, P., Kristjansson, J. K. & Thauer, R. K. Kinetic mechanism for the ability of sulfate reducers to out-compete methanogens for acetate. Arch. Microbiol. 132, 285–288 (1982).
Wörner, S. et al. Gypsum amendment to rice paddy soil stimulated bacteria involved in sulfur cycling but largely preserved the phylogenetic composition of the total bacterial community. Environ. Microbiol. Rep. 8, 413–423 (2016).
Saenjan, P., Ro, S. & Vityakon, P. Methane fluxes and rice yields as a function of sulfate fertilizer with incorporated rice stubble. Asia Pac. J. Sci. Technol. 20, 337–345 (2015).
Seitaj, D. et al. Cable bacteria generate a firewall against euxinia in seasonally hypoxic basins. Proc. Natl. Acad. Sci. USA 112, 13278–13283 (2015).
Lu, Y., Wassmann, R., Neue, H.-U. & Huang, C. Dynamics of dissolved organic carbon and methane emissions in a flooded rice soil. Soil Sci. Soc. Am. J. 64, 2011–2017 (2000).
Cummings, B., Caldwell, D., Gould, D. & Hamar, D. Identity and interactions of rumen microbes associated with dietary sulfate-induced polioencephalomalacia in cattle. Am. J. Vet. Res. 56, 1384–1389 (1995).
Habtewold, J. et al. Reduction in methane emissions from acidified dairy slurry is related to inhibition of Methanosarcina species. Front. Microbiol. 9, 2806 (2018).
Ye, R. et al. pH controls over anaerobic carbon mineralization, the efficiency of methane production, and methanogenic pathways in peatlands across an ombrotrophic–minerotrophic gradient. Soil Biol. Biochem. 54, 36–47 (2012).
Wang, Z. P., DeLaune, R. D., Patrick, W. H. & Masscheleyn, P. H. Soil redox and pH effects on methane production in a flooded rice soil. Soil Sci. Soc. Am. J. 57, 382–385 (1993).
Myrbo, A. et al. Sulfide generated by sulfate reduction is a primary controller of the occurrence of wild rice (Zizania palustris) in shallow aquatic ecosystems. J. Geophys. Res. Biogeosci. 122, 2736–2753 (2017).
Williams, J. F., Mutters, R. G. & Greer, C. A. Rice Nutrient Management in California (UCANR Publ., Publ. 3516, Oakland, CA, 2010).
Schauer, R. et al. Succession of cable bacteria and electric currents in marine sediment. ISME J. 8, 1314–1322 (2014).
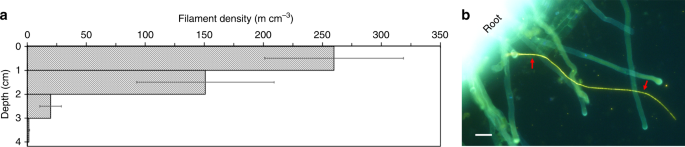
Scholz, V. V., Müller, H., Koren, K., Nielsen, L. P. & Meckenstock, R. U. The rhizosphere of aquatic plants is a habitat for cable bacteria. FEMS Microbiol. Ecol. 95, fiz062 (2019).
Martin, B. C. et al. Oxygen loss from seagrass roots coincides with colonisation of sulphide-oxidising cable bacteria and reduces sulphide stress. ISME J. 13, 707–719 (2018).
Larsen, M. et al. O2 dynamics in the rhizosphere of young rice plants (Oryza sativa L.) as studied by planar optodes. Plant Soil 390, 279–292 (2015).
Revsbech, N. P. & Jørgensen, B. B. in Advances in Microbial Ecology, Vol. 9, 293–352 (Springer, Boston, 1986).
Source: Ecology - nature.com


