Independent monophyletic origin of Culex pipiens pipiens and Culex pipiens molestus
Two alternative hypotheses have been proposed to explain the differences in ecological and physiological strategies of Cx. p. pipiens and Cx. p. molestus19. One hypothesis explains such differentiation as a rapid shift in physiological and behavior traits as an adaptation to an underground environment that is associated with human activities22. In this scenario, the local populations of Cx. p. molestus must be closely related to the local populations of Cx. p. pipiens30. This scenario considers Cx. p. molestus as an eco-physiological variant of Cx. p. pipiens. An alternative hypothesis suggests that the difference between Cx. p. pipiens and Cx. p. molestus is a result of the distinction in their evolutionary history. Ecological and physiological strategies of Cx. p. molestus could have first arisen under the warm climate19. Later, such a strategy appeared to be relevant because of the human activities that created an underground environment and, thus, spread Cx. p. molestus all over the world. This scenario considers Cx. p. molestus and Cx. p. pipiens as separate evolutionary entities.
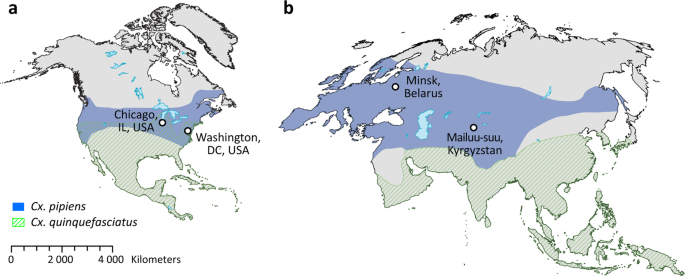
In our study, we tested these hypotheses using whole genome analysis of 40 Cx. p. molestus and Cx. p. pipiens samples collected from four locations in Eurasia and North America. Our findings rejected the hypothesis of reemergence of Cx. p. molestus from the local populations of Cx. p. pipiens and strongly supported the idea of an independent monophyletic origin of both Cx. p. pipiens and Cx. p. molestus from different continents. NJ and ML analyses separately cluster Cx. p. pipiens and Cx. p. molestus from the Republic of Belarus, the Kyrgyz Republic, and Chicago, IL, USA (Fig. 2, Supplementary Figs. 1–3). The presence of an additional hybrid cluster formed by autogenic and anautogenic field collected specimens from Washington, D.C., USA more likely reflects the existence of a Cx. pipiens – Cx. quinquefasciatus hybrid zone in this area16. However, based on genetic distances, this cluster is much closer related to the Eurasian Cx. p. pipiens rather than to the Cx. p. molestus from Eurasia and the USA (Chicago, IL). Additionally, PCA (Fig. 3) and ADMIXTURE (Fig. 4) analyses identified two distinct genetic clusters that correspond to Cx. p. pipiens and Cx. p. molestus. In both cases, all the specimens from Washington, D.C. clustered together with Cx. p. pipiens from other locations. Genome-wide pairwise Fst values were highly significant for all the comparisons (Fig. 5). We did not identify any specific region of high divergence in the genome (Fig. 6). Overall, Fst values were much higher between Cx. p. molestus and Cx. p. pipiens than between autogenic and anautogenic samples of Cx. p. pipiens from Washington, D.C. The most diverged group, based on all conducted analyses, was the strain of Cx. p. molestus from Chicago, IL, which can be explained by the very low genetic diversity of this strain caused by its longtime colonization and possible bottleneck, which accelerated genetic drift (Fig. 7).
Similar observations about the independent monophyletic origin of Cx. p. molestus and Cx. p. pipiens were obtained using microsatellite analysis in ~600 samples of Cx. pipiens mosquitoes derived from different world-wide locations19. The unrooted distance tree procedure clustered aboveground populations of Cx. p. pipiens and underground populations of Cx. p. molestus from northern and southern Europe separately. Similar to our study, the aboveground populations of Cx. p. pipiens from the USA formed an additional cluster suggesting more intense hybridization between the members of the Cx. pipiens complex in North America. The admixture analysis indicated the presence of three major clusters that corresponded to Cx. p. molestus, Cx. p. pipiens, and Cx. quinquefasciatus. The latter cluster was found in this study only in the USA samples. Another work based on amplified fragment length polymorphism (AFLP) analyses compared samples from southern and northern Europe and strains from Chicago, IL, USA24. The North American and European populations used in this study showed a similar ADMIXTURE pattern in the AFLP genome scan. The analysis of COI genes also indicated a monophyletic origin of Cx. p. pipiens and Cx. p. molestus in Europe, Asia, and Africa26.
Thus, the independent monophyletic origin and high level of genetic divergence between Cx. p. molestus and Cx. p. pipiens suggest that these two members of the Cx. pipiens complex represent distinct phylogenetic entities with independent evolutionary histories prior to human-mediated translocation.
Concepts of speciation and evolution of the Culex pipiens complex
Theoretical models of speciation in animals could be subdivided into two major groups: allopatric or geographical speciation and sympatric or ecological speciation. The first concept of speciation, which was intensively promoted by E. Mayr3, suggests that incipient taxa first become isolated geographically. This situation reduces the gene flow between the populations and may lead to accumulation of mutations that cause genetic incompatibility among the hybrids. An alternative concept of speciation emphasizes ecological barriers between the emerging taxa as major drivers of evolution31. This scenario considers the development of reproductive isolation between the populations as a result of adaptation to different environments without geographical isolation, which usually takes place in the face of gene flow. In this situation, the hybrids between incipient taxa become less fit to the environment that promotes natural selection of any traits that reduce mating between them. We think that overall diversification of the Cx. p. pipiens and Cx. p. molestus subspecies represents a striking example of speciation through isolation-by-ecology mechanisms. In our study, the Fst analysis determined significant levels of genomic divergence between Cx. p. pipiens and Cx. p. molestus (Figs. 5 and 6) across the entire genome without clear islands of speciation. Surprisingly, the levels of differentiation were lower around the centromeres, probably due to the low number of reliable SNVs in these highly repetitive regions. The differentiation was extremely high between the Chicago, IL, USA strains of Cx. p. pipiens and Cx. p. molestus, but was lower between the subspecies in the Eurasian mosquito collections. Overall, these observations suggest significant restriction of gene flow between the subspecies.
Several mechanisms of reproductive isolation between Cx. p. pipiens and Cx. p. molestus have been described17. Two of them are prezygotic acting, before fertilization, and reduce the opportunities for mosquito mating. The first mechanism is related to habitat specialization of the larvae: Cx. p. molestus occupies basements or other underground environments, but Cx. p. pipiens prefers open aboveground water bodies for breeding sites. This reduces chances for the two subspecies to meet and mate at the adult stages. The second isolating mechanism relies on the differences in mating behavior between Cx. p. molestus and Cx. p. pipiens. Cx. p. molestus males usually form homogeneous swarms near the ground and require limited space for mating17. In contrast, Cx. p. pipiens males swarm near the foliage about 2–3 m above the ground. Experimental studies of mating behavior in small cages indicated that in crosses of females and males of Cx. p. molestus copulation success was 90% but in Cx. p. pipiens it was only 3.3%32. In inter-subspecies crosses between Cx. p. molestus and Cx. p. pipiens, the copulation success was also low and varied between 6.6% to 10% depending on the sexes of the subspecies. This study demonstrated that females of both subspecies actively avoid copulation with males from an alternative subspecies. Moreover, Cx. p. pipiens females were unsuccessful in receiving sperm from Cx. p. molestus and, as a result, produced no eggs.
Two other reproductive isolating mechanisms described for Cx. p. pipiens and Cx. p. molestus are postzygotic, they act after the mating and result in decreased fitness of the hybrids. One of the mechanisms is related to the inheritance of diapause in hybrids of Cx. p. molestus and Cx. p. pipiens as a recessive trait8. F1 hybrids and a significant portion of F2 hybrids are unable to develop diapause and cannot survive under winter conditions. This mechanism, perhaps, could explain the higher introgression levels in Cx. p. pipiens in southern locations19,23,24. Finally, the members of the Cx. pipiens complex are exposed to cytoplasmic incompatability of hybrids infected with different strains the rickettsial parasite Wolbachia pipientis. Despite cytoplasmic introgression of this parasite through hybridization between the members of the Cx. pipiens complex33, cytoplasmic incompatibility could significantly limit survival rates of the hybrids. For example, W. pipientis infection significantly reduced hybridization between Cx. pipiens and Cx. quinquefasciatus in South Africa34. Another study demonstrated that in Eurasian populations Cx. p. molestus was only infected by one strain of W. pipientis but Cx. p. pipiens by two different strains35. Moreover, the specimens of Cx. p. molestus and Cx. p. pipiens, which we used in our study, were infected with the same strains of W. pipientis in the south of the Kyrgyz Republic but by different strains in the north of the Republic of Belarus. Thus, it may explain the differences in introgression from Cx. p. molestus to Cx. p. pipiens that was more pronounced in the Republic of Belarus than in the Kyrgyz Republic. In fact, an interesting example of infectious speciation was described in the South American Drosophila paulistorum complex. In this complex, six semi-species with overlapping geographic distribution became reproductively isolated as a result of premating and postmating isolation triggered by the Wolbachiae infection36.
Recent genomic studies conducted on different organisms including Drosophila simulans37, Rhagoletis fruit flies38,39, and Heliconius butterflies40,41 provide additional evidence that ecological speciation occurs in nature. The genomic patterns of speciation can be very different2 ranging from small genomic islands of speciation40 to significant levels of divergence across the entire genome. Widespread genomic divergence was identified between the incipient species Anopheles gambiae and An. coluzzii in the An. gambiae complex42. These species were originally identified by differences in the structure of their ribosomal DNA as S and M forms43, but later their taxonomic status was elevated to the species level44. An. gambiae and An. coluzzii are believed to develop reproductive barriers in sympatry as a result of the differences in their ecological preferences45. The An. gambiae larval stage is associated with small rain pools. In contrast, An. coluzzi exploits persistent water reservoirs associated with rice cultivation. Although, premating barriers between the species are incomplete46, they developed differences in their swarming behavior47,48 and divergent song types49.
Thus, a high level of genome-wide divergence, a striking difference in adaptation to distinct ecological environments and evidence of prezygotic and postzygotic barriers for mating suggest that Cx. p. pipiens and Cx. p. molestus represent distinct ecological units that undergo incipient ecological speciation.
Hybridization in the Culex pipiens complex
The most intriguing observation of the members of the Cx. pipiens complex is that, despite differences in ecology, physiology, behavior, and geographic distribution, they still can hybridize and produce viable progeny in nature, indicating that reproductive isolation between them is not complete. Our study also demonstrated significant hybridization events between the subspecies Cx. p. molestus and Cx. p. pipiens. Whole genome comparison indicated that most of the Cx. p. pipiens samples represent individuals with some level of introgression from Cx. p. molestus (Fig. 4). We observed high discrepancy between the nuclear and mitochondrial phylogenies (Figs. 2, 8), which indicates that, historically, the transmission of mitochondrial genomes can happen between the subspecies. At the same time, we did not observe haplogroups shared between the local populations of the subspecies. In the continents, all the mitochondrial phylogenies were strongly monophyletic, which points to male-mediated dispersal and hybridization. The admixture with Cx. p. molestus was higher in southern populations of Cx. p. pipiens in the Kyrgyz Republic and in Washington, D.C., USA, but lower in northern populations in the Republic of Belarus and Chicago, IL, USA. This could be related to an incapability of the hybrids to develop diapause in cold climates. For comparison, microsatellite analysis revealed a modest level of hybridization between Cx. p. pipiens and Cx. p. molestus of ~8% in the northern cities of the USA (Chicago, IL and New York, NY)30,50. In southern Europe, where Cx. p. pipiens and Cx. p. molestus could both be found aboveground, the hybridization levels between them were similar and was estimated at 8–10%51. Much higher levels of hybridization were found in southern populations in eastern USA, where ~40% of all samples were identified as hybrids between Cx. p. molestus and Cx. p. pipiens19. Thus, overall hybridization rates between the members of Cx. pipiens were higher in North America than in the Old World. For comparison, hybridization between cryptic species in the An. gambiae complex (An. gambiae and An. coluzzi) significantly varied between 1% in Mali46 to >20% in Guinea Bissau52, which is comparable to overall hybridization levels between Cx. p. molestus and Cx. p. pipiens.
Intriguingly, in our study, we did not determine a Cx. p. pipiens admixture signature in any Cx. p. molestus samples from the Republic of Belarus, the Kyrgyz Republic, and Chicago, IL, USA (Fig. 4). These findings demonstrate very limited or no gene flow from Cx. p. pipiens to Cx. p. molestus. Similar findings of asymmetric introgression from Cx. p. molestus to Cx. p. pipiens was shown by previous studies20,51. The mechanism of asymmetric introgression is currently unknown. One hypothesis suggests that males of Cx. p. molestus, which can mate in confined spaces, can hybridize with both Cx. p. molestus and Cx. p. pipiens females. In contrast, Cx. p. pipiens males, which require space for swarming, have a higher disposition to mate with Cx. p. pipiens females51. However, more population genetic and experimental studies are needed to explain this phenomenon.
Finally, our study demonstrated that Cx. p. pipiens can develop autogeny as a result of adaptive introgression of the genetic material from Cx. p. molestus. In the field collected samples from aboveground and underground environments in Washington, D.C., USA, we selected mosquitoes for autogeny. The underground mosquitoes were autogenic but the aboveground mosquitoes were anautogenic. However, mosquitoes from both autogenic and anautogenic colonies formed a single cluster when neighbor joining analysis was applied (Fig. 2). Moreover, PCA (Fig. 3) and ADMIXTURE (Fig. 4) approaches cluster these samples together with Cx. p. pipiens from other locations. Populations with mixed characteristics were found in Europe51 and in the USA53. In Portugal, an unusual pattern of blood feeding behavior on birds was also found in Cx. p. molestus54.
Thus, the presence of ongoing hybridization between members of the Cx. pipiens complex suggests that the speciation process between them is not complete and postzygotic barriers of reproductive isolation are not fully formed. Overall, we believe that members of the Cx. pipiens complex represent a remarkable model for studying different aspects of geographical and ecological speciation in the face of ongoing gene flow between them and local adaptations to diverse environments.
Source: Ecology - nature.com



