Selective carbon sources influence the end products of nitrate respiration in microbial enrichment cultures
To enrich for nitrate-respiring microbial communities from samples of aquatic sediment, agricultural soil, and groundwater, we inoculated them into a medium with 20 mM sodium nitrate as the electron acceptor and 2 g/L yeast extract as the carbon source and electron donor, and we grew them anoxically at 30 °C (“Materials and methods”). We minimized passaging of the enrichments in an effort to preserve as diverse a community as possible. Each enrichment was cryopreserved, recovered in media with yeast extract, washed, and subsequently cultured in the presence of 94 different carbon sources. Growth, pH, nitrite, and ammonium concentrations were measured after 48 h. Because our growth medium contains ammonium as a nitrogen source, we corrected for ammonium assimilated into biomass using conversion factors based on assumptions about the percentage of nitrogen in biomass and measurements of optical density (“Materials and methods”). This correction has only a minor impact on the relative ranking of carbon sources in terms of ammonium production, but for some carbon sources our estimates suggest that more ammonium was consumed than produced by the microbial community (Fig. 2).
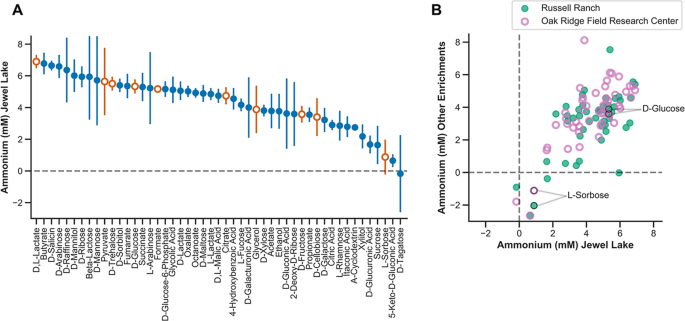
a Ammonium production (mM) of the Jewel Lake enrichment cultured on 48 diverse sugars, organic acids, and alcohols. Symbols represent means and error bars represent the standard deviation of four replicates. Orange, open symbols are carbon sources selected for further 16S microbial community analysis, isolations, and metagenomics. b Mean ammonium produced (mM) by cultures cultured on the 48 diverse carbon sources (shown in a) compared between the Jewel Lake enrichment and the Russell Ranch (closed symbols) or Oak Ridge Field Research Center enrichments (open symbols).
We were primarily interested in identifying carbon sources that influence the end products of nitrate respiration because they are specifically utilized by microbial subpopulations with different respiratory pathways. As such, we were concerned that (1) ammonium might be released from some nitrogen-containing carbon sources (2), low pH toxicity might select against some strains, or (3) optical density measurements used to estimate ammonium assimilated into biomass might be skewed by compound precipitation. Thus, we excluded from further analysis those carbon sources that (1) contain a nitrogen atom that can be released through microbial catabolism (2), lead to a pH < 5 after 48 h of growth, or (3) resulted in a measurable optical density in the absence of microbial growth. In general, the carbon sources we excluded based on these criteria produced a similar range of ammonium concentrations as those we pursued in more depth (Table S1), but we expect them to have indirect effects on ammonium production and community composition aside from selecting for strains with distinct carbon catabolic and nitrate respiratory pathways. For example, in cultures amended with some amino acids and nucleotides, ammonium production was higher than was possible via reduction of the 20 mM nitrate in our growth medium alone. This is likely because ammonium is released via catabolic deamination of these nitrogen-containing carbon sources. Thus, to avoid this complicating activity, we focused on a subset of 48 carbon sources including organic acids, alcohols and sugars for further analysis (Fig. 2).
We compared the ammonium concentrations in cultures grown on different carbon sources and identified carbon sources with a consistent influence on ammonium production within a single enrichment (Fig. 2a) and between enrichments (Fig. 2b). For example, L-sorbose reproducibly drives less ammonium production compared with D-glucose across replicates in the JL enrichment (Fig. 2a). A similar result was observed for the other two enrichments (Fig. 2b, Supplementary Table S1).
There is no obvious relationship between the chemical class of carbon source and ammonium production, as both organic acids and sugars are distributed across the range of ammonium concentrations we observed (Fig. 2a). Because all carbon sources were added at a concentration of 20 mM, this represents an electron equivalent excess of donor relative to acceptor (20 mM nitrate) in nearly every case regardless of whether we consider the five electron reduction of nitrate to dinitrogen or eight electron reduction of nitrate to ammonium. Thus, we expect that these growth conditions should tend to favor DNRA [3,4,5,6,7]. As such, the clear difference in ammonium production between carbon sources with similar electron donor equivalencies demonstrates a selective influence of the carbon source on nitrate reduction end products rather than an influence of carbon to nitrate ratio. For example, both D-glucose and L-sorbose provide 24 electron equivalents per mole, but D-glucose consistently led to more ammonium production (Fig. 2).
Microbial community compositional shifts associated with different end products of nitrate respiration
We hypothesized that the difference in ammonium production between enrichment cultures recovered on different carbon sources could be attributed to differences in the composition and gene content of the nitrate-respiring microbial communities selectively enriched on each carbon source. To understand the relationship between ammonium production and microbial community composition, we cultured the JL enrichment in triplicate on ten carbon sources that produced varying levels of ammonium (open symbols in Fig. 2a) and measured pH, optical density, nitrite, ammonium, and microbial community composition by 16S rDNA amplicon sequencing.
We measured correlations between ammonium, nitrite, optical density and pH across the enrichment cultures (Fig. 3a, b). Nitrite and ammonium concentrations are negatively correlated with each other across cultures (Pearson’s correlation r = −0.58, p = 0.00072) (Fig. 3a, b). Also, higher nitrite concentrations are associated with lower growth (Fig. 3b). This is consistent with the fact that nitrate reduction to nitrite yields less energy than nitrate reduction to ammonium or dinitrogen.
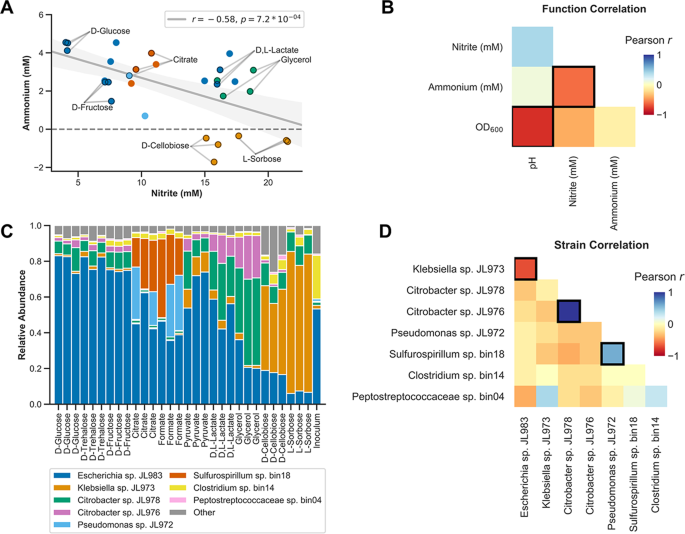
a Ammonium and nitrite concentrations for the Jewel Lake enrichment cultured on ten different carbon sources in triplicate. Points are colored based on which dominant strain is most highly selectively enriched in each condition (see c legend). Dominant strains are 16S rDNA exact sequence variants (ESVs/strains) observed at a relative abundance of >5% in any culture. Linear fit (Pearson correlation) of the nitrite and ammonium data is displayed (gray line) as well as the estimated 95% confidence interval (light gray shading) and linear correlation using Pearson’s r (legend). b Pearson correlations between functional activity measurements for the Jewel Lake enrichment cultured on the same ten carbon sources from a. Significant correlations, where p < 0.05 and Benjamini–Hochberg false discovery rate (FDR) q values were <0.1, are indicated by bold borders. c Relative abundances of strains in the Jewel Lake enrichment cultured on different carbon sources. Coloring as in a. d Pearson correlations between the relative abundances of strains in the Jewel Lake enrichment. Significant correlations, after FDR correction, are indicated by bold borders.
The electron donor equivalents per molecule provided by this set of ten carbon sources varies from 6 electrons for formate to 44 electrons for trehalose. Thus, in most cases there is an electron equivalent excess of carbon relative to nitrate (NO3− to N2 is five electrons, NO3− to NH4+ is eight electrons). We observed no significant correlations between electron donor equivalents and ammonium or nitrite concentrations (Fig. S1A, B). We also observed no significant correlations between pH and ammonium or nitrite concentrations (Fig. S1C, D). However, lower pH is associated with more growth which is consistent with organic acid production through fermentation of the sugars (Fig. 3b). It is known that fermentation can compete with nitrate respiration to influence nitrate respiratory end products [3, 48], but in our enrichments this is not a dominant factor.
To understand the mechanistic basis for these correlations we obtained pure cultures and sequenced the genomes of several dominant 16S rDNA ESVs by plating the JL enrichment on anaerobic agar plates amended with carbon sources and nitrate. We also sequenced metagenomes of carbon-source enrichments that were dominated by ESVs that we did not isolate. We matched each 16S rDNA ESV with 16S rDNA sequences in genome sequenced isolates. For ESVs we did not isolate, we matched the SILVA [40] taxonomy of ESVs with the GTDB-Tk [39] taxonomy of the most closely related MAG with the highest fold coverage. For these metagenomes from low-complexity enrichments, there is no ambiguity about which MAG corresponds to which 16S ESV. Thus, we are able to track the abundance of specific strains in the JL enrichment with known genetic potential across cultures using 16S rDNA amplicons.
We observed specific strains enriched on different carbon sources (Fig. 3c, Fig. S2). This is consistent with our hypothesis that selective carbon sources alter the composition of the microbial community and thereby influence nitrite and ammonium production. We focused on strains that are present at >5% relative abundance in any of the enrichment cultures and measured correlations between these strains (Fig. 3d). The Escherichia and Klebsiella strains are strongly negatively correlated with each other (Fig. 3d). The Escherichia strain is dominant in D-glucose, D-fructose and D-trehalose while the Klebsiella is dominant in L-sorbose and D-cellobiose (Fig. 3c). The two Citrobacter strains are co-enriched on D,L-lactate and glycerol, while Pseudomonas and Sulfurospirillum are co-enriched on citrate and formate. Clostridium and Peptostreptococcaceae are below 5% relative abundance in most samples, but are more abundant in the primary yeast extract enrichment, likely because they are specialists in peptide and amino acid catabolism (Fig. 3c, Table S3).
Correlations between microbial community genetic functional potential and functional activity
We identified correlations between the relative abundances of the dominant strains and pH, OD 600, nitrite, or ammonium (Fig. 4a). From the metagenomic and isolate genome sequencing we know the genetic potential of all dominant strains (Fig. 4b, Tables S2–S4). In most cases, the strains that are positively correlated with ammonium production or nitrite production have the genetic potential to carry out that function (Fig. 4a, b Tables S2, S3). For example, the Escherichia strain, whose abundance is positively correlated with ammonium production across our enrichments (Pearson correlation, r = 0.77, p < 0.0001), has the complete pathway for DNRA. In contrast, the Klebsiella strain, which is positively correlated with nitrite accumulation (Fig. 4a), has a NarG-type nitrate reductase, but no downstream enzymes involved in DNRA or denitrification and is thus predicted to be a nitrite accumulator (Fig. 4b). The Sulfurospirillum, Pseudomonas, and Citrobacter strains are weakly positively correlated with ammonium production, and with the exception of the Pseudomonas, all have the capacity for nitrate ammonification. The only strain with a complete denitrification pathway is the Pseudomonas, but the Sulfurospirillum has a nitrous oxide reductase (nosZ) and thus may participate in the final step of denitrification as well as DNRA.
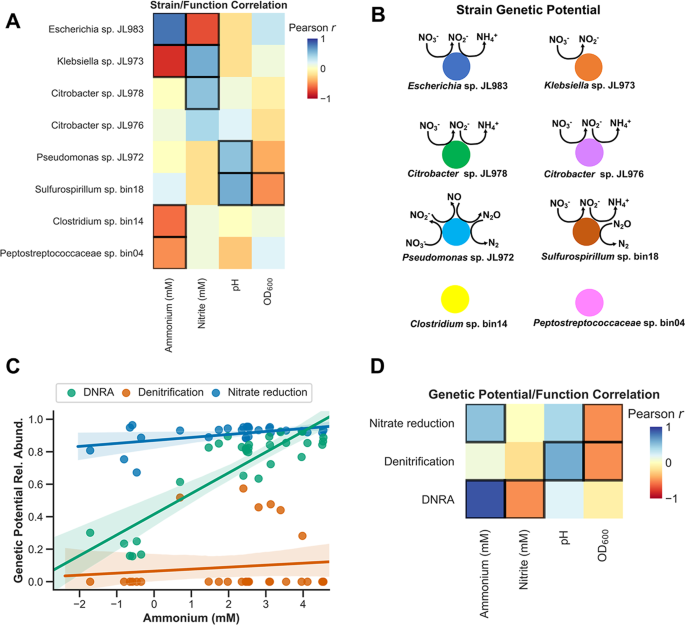
a Pearson correlations between strain relative abundances and measurements of nitrite, ammonium, pH, and OD 600. Significant correlations, after FDR correction, are indicated by bold borders. b The predicted genetic potential of each strain in the Jewel Lake enrichment to catalyze steps in nitrate reduction, DNRA, and denitrification. Coloring as in Fig. 3c. c Relative abundances of the total genetic potential for nitrate reduction, DNRA, or denitrification reduction plotted against ammonium concentrations. Each point represents a different culture. Genetic potential for each trait is the presence of genes essential for each trait. Total genetic potential is the sum of the relative abundances of each strain with each trait. d Pearson correlations between the relative abundances of the genetic potential for DNRA, denitrification or nitrate reduction and the measurements of nitrite, ammonium, pH, and OD 600. Significant correlations, after FDR correction, are indicated by bold borders.
For each culture for which we have community composition data from 16S rDNA amplicons, we can sum the relative abundance of all strains possessing the genetic potential for nitrate reduction, DNRA or denitrification to estimate the total genetic potential for each of these nitrate respiratory traits. We observe that the genetic potential for DNRA is positively correlated with ammonium production and negatively correlated with nitrite production (Fig. 4c, d). This is largely driven by changes in the relative abundance of the dominant nitrate ammonifying Escherichia relative to the nitrite accumulating Klebsiella, but the Citrobacter and Sulfurospillum strains also contribute to DNRA genetic potential (Table S2).
Specific carbon sources influence nitrate respiration end products by selectively enriching for microbial subpopulations with distinct functional traits
To understand the basis for the selective enrichment of specific strains on different carbon sources, we profiled the carbon utilization capability of several isolates derived from the JL enrichment culture. The highest fold enrichment of strains relative to the dominant Escherichia sp. JL983 is for carbon sources that the enriched strains are uniquely capable of utilizing (Fig. 5a). For example, Klebsiella sp. JL973 is the only isolated strain able to grow on L-sorbose or D-cellobiose and this strain is therefore highly enriched on these substrates. Similarly, the Pseudomonas sp. JL972 is the only isolated strain able to grow on citrate or formate and is likewise enriched on these substrates (Fig. 5a).
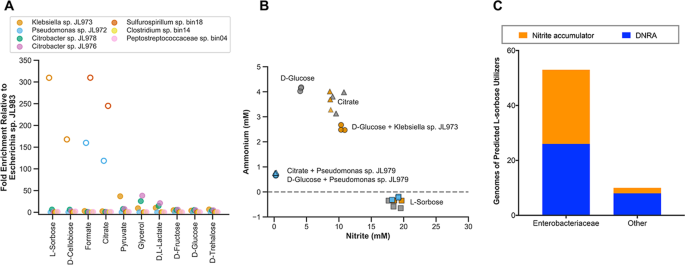
a Fold enrichment of strains relative to Escherichia in the Jewel Lake enrichment. Open symbols are strains that use carbon sources the Escherichia cannot utilize. b Nitrite and ammonium concentrations of the Jewel Lake enrichment alone (dark gray symbols) or bioaugmented with Klebsiella (orange symbols) or Pseudomonas (blue symbols) with D-glucose, citrate or L-sorbose as the sole carbon source. c For genomes that encode the L-sorbose utilization genes (sorABE), we show how often they are expected to be nitrite accumulators or nitrate ammonifiers (DNRA) based on the presence or absence of respiratory nitrate reductase genes (napA, narG) and respiratory nitrite reductase genes (nirS, nirK, nrfA). Because L-sorbose utilization is best studied in Enterobacteriaceae, we show results separately for this group than for other prokaryotes (Other). We found no predicted dentrifiers that have the sorABE genes. Data are from 27,941 prokaryotic genomes on Annotree (“Materials and methods”).
To confirm the functional traits of the strains in the JL enrichment and demonstrate the selectivity of different carbon sources in influencing community functional outcomes, we bioaugmented the JL enrichment with the Klebsiella sp. JL973 or Pseudomonas sp. JL972 strains at a 1:1 ratio of isolate to enrichment (Fig. 5b). As expected, bioaugmentation with the Klebsiella strain shifts end products toward more nitrite production and less ammonium production on D-glucose. There is no influence of Klebsiella sp. JL973 bioaugmentation on nitrate reduction end products in L-sorbose cultures because Klebsiella sp. JL973 is already dominant on this carbon source and nitrate is stoichiometrically converted to nitrite.
There was no influence of Klebsiella sp. JL973 bioaugmentation in citrate cultures, because the Klebsiella strain does not utilize citrate. In contrast, Pseudomonas bioaugmentation in D-glucose and citrate cultures shifts end products towards lower nitrite and ammonium production. This is consistent with the Pseudomonas strain’s capacity for complete denitrification and utilization of these carbon sources. While difficult to predict based on gene content, citrate utilization is heterogeneously distributed within the Enterobacteriaceae [11, 12], and anaerobic oxidation of citrate to formate by Enterobacterial isolates is generally not coupled to growth [49]. In contrast, Pseudomonas and Sulfurospirillum are generally capable of utilizing citrate anaerobically coupled to nitrate reduction [50].
We also looked for the presence of L-sorbose utilization genes (sorABE) in the genomes of organisms predicted to be denitrifiers, nitrate ammonifiers or nitrite accumulators based on the presence or absence of nitrate reductase (narG, napA) and nitrite reductase (nirS, nirK, nrfA) genes (Fig. 5c). The function of the sorABE genes has mostly been studied in the Enterobacteriaceae [47], and there may be other catabolic pathways for L-sorbose. However, a roughly equivalent number of predicted nitrate ammonifying (DNRA) and nitrite accumulating Enterobacteriaceae have sorABE. Thus, the enrichment of Enterobacterial nitrite accumulators, such as Klebsiella sp. JL973, may be a likely outcome after L-sorbose amendment in terrestrial environments. Taken together, our results demonstrate a selective influence of carbon sources in altering nitrate reduction end products in the JL enrichment by enriching for strains with specific carbon catabolic and nitrate respiratory traits.
Source: Ecology - nature.com



