Insect material
Mummified fruits containing diapausing larvae of Eurytoma maslovskii were collected from Korean apricot (Prunus mume) orchards in Suncheon, Korea (34.9 N, 127.4E) in January and February 2016, 2017, and 2018. The infested fruits were placed in screen cages (30 × 30 × 30 cm), and maintained at 25 °C under a photoperiod of L14:D10. Newly emerged adults were collected daily, and the sexes were separated based on the presence or absence of a long ovipositor on the ventral side of the abdomen. The adults were kept individually in plastic bottles (7 cm height, 2.5 cm diameter) and provided with a cotton pad soaked with a 10% sucrose solution.
Chemicals
Racemic 3-methyltridecyl propionate (90% purity), 11-methyltridecyl propionate (91% purity), 2,10-dimethyldodecyl propionate (2,10-DiMe-12Pr) (99% purity), 4,10-dimethyldodecyl propionate (91% purity), 6,10-dimethyldodecyl propionate (92% purity), 3,7-dimethyldodecyl propionate (90% purity), 3,9-dimethyldodecyl propionate (91% purity), and 2,8-dimethyldecyl propionate (2,8-DiMe-10Pr) (99% purity) were synthesized by one of the authors (KBK). The corresponding alcohols were synthesized by bromination of the commercially available methylated alkanol or methylated alkandiol, followed by chain extension with the appropriated tetrahydropyran (THP)-protected alkylmangesium bromide37,38. The propyl esters were prepared with the corresponding alcohol and propionyl chloride. All structures were confirmed by NMR analysis and/or GC-MS.
2,10-dimethyldodecyl propionate
1H NMR (500 MHz, CDCl3) δ 3.958 (dd, J = 10.7, 5.9 Hz, 1H), 3.853 (dd, J = 10.7, 6.9 Hz, 1H), 2.332 (q, J = 7.6 Hz, 2H), 1.819–1.718 (m, 1H), 1.420–1.202 (m, 14H), 1.147 (t, J = 7.6 Hz, 3H), 1.157–1.049 (m, 3H), 0.917 (d, J = 6.8 Hz, 3H), 0.895 (t, J = 7.3 Hz, 3H), 0.839 (d, J = 6.4 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 174.65 (C), 69.33 (CH2), 36.65 (CH2), 34.42 (CH), 33.39 (CH2), 32.57 (CH), 30.00 (CH2), 29.86 (CH2), 29.66 (CH2), 29.51 (CH2), 27.67 (CH2), 27.11 (CH2), 26.84 (CH2), 19.23 (CH3), 16.90 (CH3), 11.42 (CH3), 9.22 (CH3).
2,8-dimethyldecyl propionate
1H NMR (500 MHz, CDCl3) δ 3.958 (dd, J = 10.7, 5.9 Hz, 1H), 3.854 (dd, J = 10.7, 6.9 Hz, 1H), 2.332 (q, J = 7.6 Hz, 2H), 1.820–1.705 (m, 1H), 1.412–1.214 (m, 10H), 1.146 (t, J = 7.6 Hz, 3H), 1.157–1.048 (m, 3H), 0.918 (d, J = 6.7 Hz, 3H), 0.854 (t, J = 7.3 Hz, 3H), 0.840 (d, J = 6.3 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 174.64 (C), 69.32 (CH2), 36.60 (CH2), 34.41 (CH), 33.40 (CH2), 32.58 (CH), 30.19 (CH2), 29.51 (CH2), 27.67 (CH2), 27.04 (CH2), 26.87 (CH2), 19.23 (CH3), 16.91 (CH3), 11.41 (CH3), 9.22 (CH3).
Moreover, all four possible enantiomers (>99% ee) of 2,10-DiMe-12Pr and 2,8-DiMe-10Pr used in field bioassays were synthesized by one of the authors (KM). Details of the syntheses of these chiral compounds will be described elsewhere (Okubo et al., in press).
Pheromone extraction
Preliminary observations revealed that E. maslovskii usually mates in the field during the morning, usually at 0900–1200 h. Males were captured in traps baited with female thoraces without heads or abdomens, as in other eurytomid wasp20. For this, 1–2-d-old females were sectioned into head, thorax and abdomen under a binocular microscope during the fourth to fifth hour of the photophase. Ten excised thoraces were immersed in 200 μl hexane in a 5-ml conical glass vial (Wheaton, Millville, NJ, USA) for 10 min and pooled to yield a crude extract. The hexane extract was then transferred into another vial and stored at −20 °C until use.
Fractionation of thoracic extracts
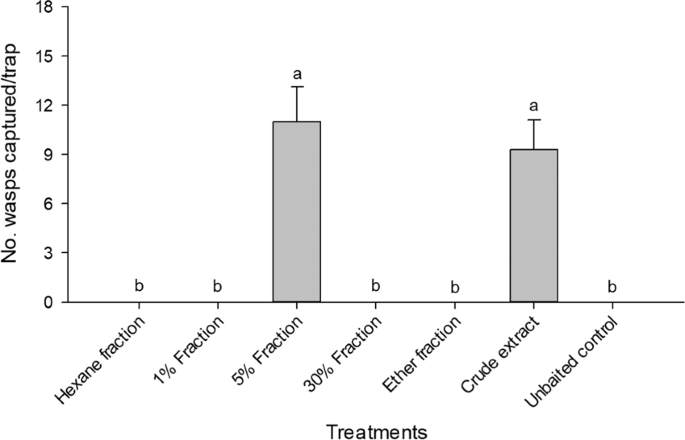
A column prepared from a Pasteur pipette plugged with a small plug of glass wool was loaded with 500 mg silica gel (Wakogel C-200, Japan), rinsed with hexane, and loaded with a thoracic extract from 500 virgin females. Substances were then eluted from the column sequentially with 4 ml hexane (hexane fraction), 4 ml 1% diethyl ether in hexane (1% fraction), 4 ml 5% diethyl ether in hexane (5% fraction), 4 ml 30% diethyl ether in hexane (30% fraction), and 4 ml diethyl ether (ether fraction). Sequential elution with hexane, diethyl ether in hexane, and diethyl ether yielded fractions containing nonpolar, modest polar and more polar compounds, in sequence. Each fraction was concentrated to 50 μl under a stream of nitrogen and kept in a freezer (−20 °C) until being used in field bioassays or chemical analyses.
Gas Chromatography-Electroantennographic Detection (GC-EAD)
Aliquots of behaviorally active fractions (5% ether in hexane) of female thoracic extracts were subjected to gas chromatography-electroantennographic detection (GC-EAD) analyses39, using an Agilent GC 7890B, equipped with a DB-5 column (30 m×0.25 mm ID, 0.25 μm film thickness; J&W Scientific, CA, USA) and an electroantennography system (Syntech, Kirchzarten, Germany). The injector and detector temperatures were 250 and 260 °C, respectively. The effluent from the GC column was split between a flame ionization detector (FID) and the antenna of a male wasp (split ratio 1:1). Hydrogen was used as the carrier gas. The GC oven was programmed from 80 °C/1 min to 250 °C at 10 °C/min and held for 10 min.
An excised male E. maslovskii antenna was positioned between two glass capillary electrodes. Each end of the antenna was embedded in electrode gel (spectra 360; Parker Laboratories, NJ, USA) and applied on electrode holders. The EAD exit port temperature was maintained at 230 °C and the antennal preparation was continuously exposed to a charcoal-filtered and humidified airstream. Antennal signals were amplified using a universal AC/DC amplifier and analyzed on a computer equipped with a signal acquisition interface board (IDAC-4, Syntech) running a GC-EAD software (AutoSpike version 3.2, Syntech).
Gas Chromatography-Mass Spectrometry (GC-MS)
Female extracts and synthetic standards were analyzed on an Agilent 6890 N GC interfaced to an Agilent 5975 C mass-selective detector as previously described40. Samples were run on a non-polar DB-5MS or polar DB-Waxetr column (30 m×0.25 mm ID, 0.25 μm film thickness; J&W Scientific, CA, USA). For the DB-5MS column, the oven temperature was identical to that previously described for the GC-EAD system. For the DB-Waxetr column, the oven temperature was maintained at 80 °C for 1 min, increased to 180 °C at 10 °C/min, then to 220 °C at 5 °C/min, and held for 10 min. The injection was splitless, and helium was the carrier gas (1 ml/min). The injector and transfer line temperatures were 250 °C. Electron ionization mass spectra were recorded from m/z 30 to 350 at 70 eV, with the ion source temperature of 230 °C. GC retention times are quoted as retention indices (RIs) relative to those of n-alkanes. Compounds from female thoracic extracts were identified by comparisons of their RIs and mass spectra with those of authentic standards on the DB-5MS and DB-Waxetr columns. Quantities of each compound in the female extracts were calculated using hexane solution (1 ng/uL) of synthetic racemic 2,10-DiMe-12Pr as an external standard.
Field trials
Field experiments were conducted in three Korean apricot orchards in Suncheon, Korea in April and May 2018 and 2019. Delta traps (Green Agro Tech, Gyeongsan, Korea) were baited with red rubber septa (Aldrich Chemical, Milwaukee, WI, USA) loaded with test compounds. Traps were hung from apricot branches approximately 1.5 m above ground. In all experiments, a randomized complete block design was used with three replicates per treatment. The distance between traps within each block was 10 m, and the distance between blocks was at least 100 m.
Experiment 1 tested biological activities of five fractions of female thoracic extracts. Traps were baited with 50 female equivalents of the fractions. In addition, traps baited with crude extracts of 50 female thoraces and traps without baits were used as controls. Experiment 2 was designed to determine which enantiomers of 2,10-DiMe-12Pr and 2,8-DiMe-10Pr are pheromone components of E. maslovskii. Treatments were racemic mixtures, (RR)-, (RS)-, (SR)-, and (SS)-enantiomers of 2,10-DiMe-12Pr and 2,8-DiMe-10Pr, respectively. Experiment 3 analyzed the potential synergism between (2S,10R)-DiMe-12Pr and (2S,8S)-DiMe-10Pr by testing them separately and in mixtures with different mixing ratios (100:0, 80:20, 50:50, 20:80, 0:100). Experiment 4 was designed to determine the antagonist effects of the other enantiomers (RR, RS, or SS) on the attraction to (2S,10R)-DiMe-12Pr. Finally, experiment 5 compared the relative attractiveness of different doses (1000, 100, 10, 1 ug) of (2S,10R)-DiMe-12Pr.
Because field trapping data did not adequately meet assumptions of normality or heteroscedasity, differences were analyzed via nonparametric Kruskal-Wallis analysis of variance (SAS Institute Inc. 2014). Post-hoc pairwise comparisons were computed using a Mann-Whitney U-test at the 5% significance level.
Source: Ecology - nature.com



