Pacifici, M. et al. Assessing species vulnerability to climate change. Nat. Clim. Change 5, 215–225 (2015).
Foden, W. B. & Young, B. E. (eds) IUCN SSC Guidelines for Assessing Species’ Vulnerability to Climate Change (IUCN Species Survival Commission, Gland, 2016).
Conde, D. A. et al. Data gaps and opportunities for comparative and conservation biology. PNAS 116, 9658–9664 (2019).
Pearson, R. G. Species’ distribution modeling for conservation educators and practitioners. Lessons Conserv. 3, 54–89 (2010).
Tsiftsis, S., Djordjević, V. & Tsiripidis, I. Neottia cordata (Orchidaceae) at its southernmost distribution border in Europe: Threat status and effectiveness of Natura 2000 Network for its conservation. J. Nat. Conserv. 48, 27–35 (2019).
Margules, C. R. & Sarkar, S. Systematic Conservation Planning (Cambridge University Press, Cambridge, 2007).
Elith, J. & Leathwick, J. The contribution of species distribution modelling to conservation prioritization. In Spatial Conservation Prioritization. Quantitative Methods and Computational Tools (eds Moilanen, A. et al.) 70–93 (Oxford University Press Inc., Oxford, 2009).
Thompson, R. N. & Brooks-Pollock, E. Detection, forecasting and control of infectious disease epidemics: Modelling outbreaks in humans, animals and plants. Philos. Trans. R. Soc. Lond. B Biol. Sci. B374, 20190038 (2019).
Bertolino, S. et al. Spatially-explicit models as tools for implementing effective management strategies for invasive alien mammals. Mamm. Rev. 50, 187–199 (2020).
Suggitt, A. J. et al. Conducting robust ecological analyses with climate data. Oikos 126, 1533–1541 (2017).
Rojas-Sandoval, J. & Meléndez-Ackerman, E. J. Spatial patterns of distribution and abundance of Harrisia portoricensis, an endangered Caribbean cactus. J. Plant Ecol. 6, 489–498 (2013).
Smeraldo, S. et al. Modelling risks posed by wind turbines and power lines to soaring birds: The black stork (Ciconia nigra) in Italy as a case study. Biodivers. Conserv. 29, 1959–1976 (2020).
Jensen, A. M., O’Neil, N. P., Iwaniuk, A. N. & Burg, T. M. Landscape effects on the contemporary genetic structure of ruffed grouse (Bonasa umbellus) populations. Ecol. Evol. 9(10), 5572–5592 (2019).
Araújo, M. B. & Luoto, M. The importance of biotic interactions for modelling species distributions under climate change. Glob. Ecol. Biogeogr. 16, 743–753 (2007).
van der Putten, W. H., Macel, M. & Visser, M. E. Predicting species distribution and abundance responses to climate change: Why it is essential to include biotic interactions across trophic levels. Philos. Trans. R. Soc. Lond. B Biol. Sci. 365, 2025–2034 (2010).
Duffy, K. J. & Johnson, S. D. Specialized mutualisms may constrain the geographical distribution of flowering plants. Proc. R. Soc. B. 284, 20171841 (2017).
Engelhardt, E. K., Neuschulz, E. L. & Hof, C. Ignoring biotic interactions overestimates climate change effects: The potential response of the spotted nutcracker to changes in climate and resource plants. J. Biogeogr. 47, 143–154 (2020).
Chase, M. W. et al. An updated classification of Orchidaceae. Bot. J. Linn. Soc. 177, 151–174 (2015).
Govaerts, R. World Checklist of Orchidaceae. Facilitated by the Royal Botanic Gardens, Kew. (2019) https://wcsp.science.kew.org/. Accessed 14 Dec 2019.
Waterman, R. J. & Bidartondo, M. I. Deception above, deception below: Linking pollination and mycorrhizal biology of orchids. J. Exp. Bot. 59, 1085–1096 (2008).
Claessens, J. & Kleynen, J. The Flower of the European Orchid. Form and Function. (Jean Claessens and Jacques Kleynen, 2011).
McCormick, M. K. & Jacquemyn, H. What constrains the distribution of orchid populations?. New Phytol. 202, 392–400 (2014).
Kull, T. & Hutchings, M. J. A comparative analysis of decline in the distribution ranges of orchid species in Estonia and the United Kingdom. Biol. Conserv. 129, 31–39 (2006).
Wotavová, K., Balounová, Z. & Kindlmann, P. Factors affecting persistence of terrestrial orchids in wet meadows and implications for their conservation in a changing agricultural landscape. Biol. Conserv. 118, 271–279 (2004).
Pfeifer, M., Wiegand, K., Heinrich, W. & Jetschke, G. Long-term demographic fluctuations in an orchid species driven by weather: Implications for conservation planning. J. Appl. Ecol. 43, 313–324 (2006).
Tremblay, R. L. Trends in the pollination ecology of the Orchidaceae: Evolution and systematics. Can. J. Bot. 70, 642–650 (1992).
Cozzolino, S. & Scopece, G. Specificity in pollination and consequences for postmating reproductive isolation in deceptive Mediterranean orchids. Philos. Trans. R. Soc. Lond. B Biol. Sci. 363, 3037–3046 (2008).
Peakall, R. et al. Pollinator specificity, floral odour chemistry and the phylogeny of Australian sexually deceptive Chiloglottis orchids: Implications for pollinator-driven speciation. New Phytol. 188, 437–450 (2010).
Jersáková, J., Johnson, S. D. & Kindlmann, P. Mechanisms and evolution of deceptive pollination in orchids. Biol. Rev. 81, 219–235 (2006).
Gaskett, A. C. Orchid pollination by sexual perspectives. Biol. Rev. 86, 33–75 (2011).
Vereecken, N. J., Dafni, A. & Cozzolino, S. Pollination syndromes in mediterranean orchids—Implications for speciation, taxonomy and conservation. Bot. Rev. 76, 220–240 (2010).
Swarts, N. D. & Dixon, K. W. Terrestrial orchid conservation in the age of extinction. Ann. Bot. 104, 543–556 (2009).
Rasmussen, N. H. Terrestrial Orchids. From Seed to Mycotrophic Plant (Cambridge University Press, Cambridge, 1995).
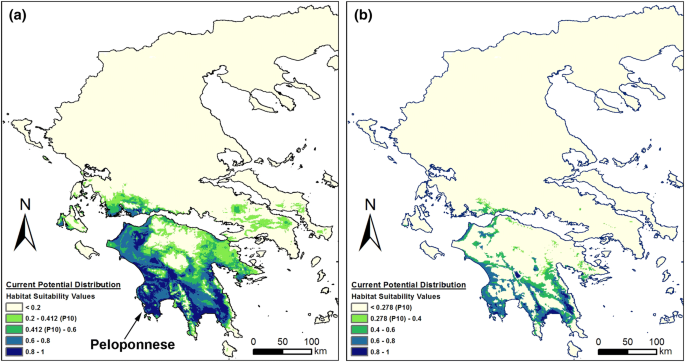
Tsiftsis, S., Tsiripidis, I. & Trigas, P. Identifying important areas for orchid conservation in Crete. Eur. J. Environ. Sci. 1, 28–37 (2011).
Tsiftsis, S., Tsiripidis, I., Trigas, P. & Karagiannakidou, V. The effects of presence/absence vs. continuous suitability data on reserve selection. Eur. J. Environ. Sci. 2, 125–137 (2012).
Kolanowska, M. Niche conservatism and the future potential range of Epipactis helleborine (Orchidaceae). PLoS ONE 8(10), e77352 (2013).
Kolanowska, M. & Rykaczewski, M. From the past to the future glacial refugia, current distribution patterns and future potential range changes of Diodonopsis (Orchidaceae) representatives. Lankesteriana 17, 315–327 (2017).
Kolanowska, M. et al. Global warming not so harmful for all plants—response of holomycotrophic orchid species for the future climate change. Sci. Rep. 7, 12704 (2017).
Štípková, Z., Romportl, D., Černocká, V. & Kindlmann, P. Factors associated with the distributions of orchids in the Zeleníky mountains, Czech Republic. Eur. J. Environ. Sci. 7, 135–145 (2017).
Martinis, A., Chaideftou, E., Minotou, C. & Poirazidis, K. Spatial analysis of orchids diversity unveils hot-spots: The case of Zante Island, Greece. J. Agric. Inform. 9, 26–40 (2018).
Paulus, H. F. & Gack, C. Pollination of Ophrys (Orchidaceae) in Cyprus. Plant Syst. Evol. 169, 177–207 (1990).
Holt, R. D. Bringing the Hutchinsonian niche into the 21st century: Ecological and evolutionary perspectives. PNAS 106, 19659–19665 (2009).
Antonopoulos, Z. & Tsiftsis, S. Atlas of the Greek Orchids Vol. II (Mediterraneo Editions, Rethymno, 2017).
Waud, M., Brys, R., Van Landuyt, W., Lievens, B. & Jacquemyn, H. Mycorrhizal specificity does not limit the distribution of an endangered orchid species. Mol. Ecol. 26, 1687–1701 (2017).
Giannini, T. C. et al. Climate change in the Eastern Amazon: Crop-pollinator and occurrence-restricted bees are potentially more affected. Reg. Environ. Change 20, 9 (2020).
Elias, M. A. S., Borges, F. J. A., Bergamini, L. L., Franceschinelli, E. V. & Sujii, E. R. Climate change threatens pollination services in tomato crops in Brazil. Agric. Ecosyst. Environ. 239, 257–264 (2017).
Burkle, L. A., Marlin, J. C. & Knight, T. M. Plant–pollinator interactions over 120 years: Loss of species, co-occurrence, and function. Science 339, 1611–1615 (2013).
Robbirt, K. M., Roberts, D. L., Hutchings, M. J. & Davy, A. J. Potential disruption of pollination in a sexually deceptive orchid by climate change. Curr. Biol. 24, 2845–2849 (2014).
Schweiger, O. et al. Increasing range mismatching of interacting species under global change is related to their ecological characteristics. Glob. Ecol. Biogeogr. 21, 88–99 (2012).
Holt, R. D. The microevolutionary consequences of climate change. Trends Ecol. Evol. 5, 311–315 (1990).
Saupe, E. E. et al. Variation in niche and distribution model performance: The need for a priori assessment of key causal factors. Ecol. Model. 237–238, 11–22 (2012).
Early, R. & Sax, D. F. Climatic niche shifts between species’ native and naturalized ranges raise concern for ecological forecasts during invasions and climate change. Glob. Ecol. Biogeogr. 23, 1356–1365 (2014).
Breitkopf, H., Onstein, R. E., Cafasso, D., Schlüter, P. M. & Cozzolino, S. Multiple shifts to different pollinators fuelled rapid diversification in sexually deceptive Ophrys orchids. New Phytol. 207, 377–389 (2015).
Schlüter, P. M. et al. Stearoyl-acyl carrier protein desaturases are associated with floral isolation in sexually deceptive orchids. PNAS 108, 5696–5791 (2011).
Bilz, M., Kell, S. P., Maxted, N. & Lansdown, R. V. European Red List of Vascular Plants (Publications Office of the European Union, Brussels, 2011).
Tsiftsis, S. & Tsiripidis, I. Threat categories of the Greek orchids. Bot. Chron. 21, 43–74 (2016).
Paulus, H. F. Zur Bestäubungsbiologie einiger Ophrys-Arten in Nordthessalien mit Beschreibung von Ophrys olympiotissa aus der Ophrys argolica–ferrum-equinum-Gruppe (Orchidaceae und Insecta, Apoidea). J. Eur. Orch. 43, 498–526 (2011).
Hennecke, M. & Munzinger, S. Ophrys subgen. Fuciflorae sect. Araniferae subsect. Argolicae. Ber. Arbeitskr. Heim. Orch. 31, 232–238 (2014).
Paulus, H. F. Deceived males—Pollination biology of the Mediterranean orchid genus Ophrys (Orchidaceae). J. Eur. Orch. 38, 303–353 (2006).
Rasmont, P. & Dehon, M. Anthophora plagiata. The IUCN Red List of Threatened Species 2015: e.T19199906A21776289. (2015). Downloaded on 06 December 2019.
Phillips, S. J. & Dudík, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 31, 161–175 (2008).
Phillips, S. J., Anderson, R. P., Dudík, M., Schapire, R. E. & Blair, M. E. Opening the black box: An open-source release of Maxent. Ecography 40, 887–893 (2017).
Elith, J. et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 29, 129–151 (2006).
Tsiftsis, S. & Antonopoulos, Z. Atlas of the Greek Orchids Vol. I (Mediterraneo Editions, Rethymno, 2017).
Hijmans, R. J., Cameron, S. E., Parra, J. L., Jones, P. G. & Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 25, 1965–1978 (2005).
Fick, S. E. & Hijmans, R. J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 37, 4302–4315 (2017).
IGME. Geological Map of Greece, 1:500,000 (IGME, Cantabria, 1983).
Habel, J. C., Teucher, M. & Rödder, D. Mark-release-recapture meets Species Distribution Models: Identifying micro-habitats of grassland butterflies in agricultural landscapes. PLoS ONE 13(11), e0207052 (2018).
Kärcher, O., Frank, K., Walz, A. & Markovic, D. Scale effects on the performance of niche-based models of freshwater fish distributions. Ecol. Model. 405, 33–42 (2019).
Phillips, S. J. Transferability, sample selection bias and background data in presence-only modeling: A response to Peterson et al. (2007). Ecography 31, 272–278 (2008).
Warren, D. L. & Seifert, S. N. Ecological niche modeling in Maxent: The importance of model complexity and the performance of model selection criteria. Ecol. Appl. 21, 335–342 (2011).
Duffy, K. J. & Jacquemyn, H. Climate change increases ecogeographical isolation between closely related plants. J. Ecol. 107, 167–177 (2019).
Wróblewska, A. & Mirski, P. From past to future: Impact of climate change on range shifts and genetic diversity patterns of circumboreal plants. Reg. Environ. Change 18, 409–424 (2018).
Zurell, D., Pollock, L. J. & Thuiller, W. Do joint species distribution models reliably detect interspecific interactions from co-occurrence data in homogenous environments?. Ecography 41, 1812–1819 (2018).
Jeschke, J. M. & Strayer, D. L. Usefulness of bioclimatic models for studying climate change and invasive species. Ann. N. Y. Acad. Sci. 1134, 1–24 (2008).
Warren, D. L., Glor, R. E. & Turelli, M. Environmental niche equivalency versus conservatism: Quantitative approaches to niche evolution. Evolution 62, 2868–2883 (2008).
Nunes, L. A. & Pearson, R. G. A null biogeographical test for assessing ecological niche evolution. J. Biogeogr. 44, 1331–1343 (2017).
Martínez-Méndez, N., Mejía, O., Ortega, J. & Méndez-de la Cruz, F. Climatic niche evolution in the viviparous Sceloporus torquatus group (Squamata: Phrynosomatidae). PeerJ 6, e6192 (2019).
ESRI. ArcGIS Desktop: Release 10 (Environmental Systems Research Institute, Redlands, 2012).
Source: Ecology - nature.com



