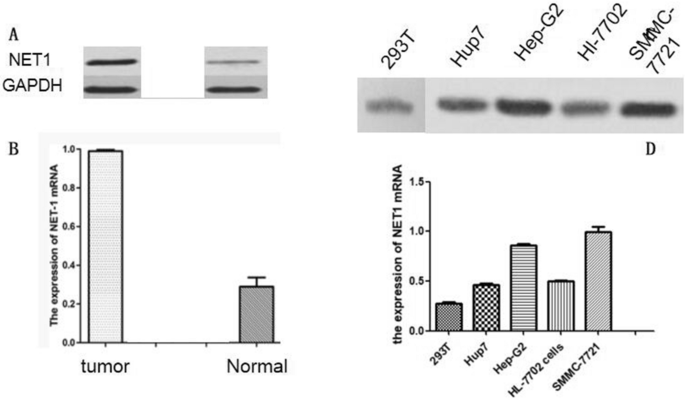
Collection of HCC tissues and maintenance of HCC cell lines
All experimental procedures were approved by the Shanghai Xuhui Hospital Ethics Committee, while human tissue experiments were conducted with the patients’ written consent. All tumor tissues were kindly provided by Shanghai Xuhui hospital. The Ethics Committee of this hospital approved all bioassays and all patients signed the written consent. All experiments were performed in accordance with Shanghai Xuhui Hospital’ guidelines and regulations. And all participants were informed consent for study participation. HEK293 cell line and hepatic cancer cell lines Hep-G2, Huh7, SMMC-7721 and HL-7702 were purchased from ATCC. All HCC cell lines were maintained and passaged in Dulbecco’s Modified Eagle’s Medium (Gibco) added with 10% fetal bovine serum (FBS; HyClone), and incubated in a warm and moisture refrigerator supplied with 5% CO2.
NET1 silencing by RNAi
The p Silencer™ siRNA expression vector (ThermoFisher) was applied to clone NET1 siRNA or its specific scramble control. The recombinant plasmids were transfected into SMMC-7721 cells using Lipofectamine 2000 (Life Technologies, USA) based on the user guidelines. 24 h later, qRT-PCR and western blot were utilized to measure the silencing effect of siRNA respectively.
Western blot
Twenty ug of total cell lysates were quantified and separated on polyacrylamide gel, and then transferred to a polyvinylidene difluoride (PVDF) membrane. Then, the PVDF membrane was preincubated with 5% nonfat dry milk prepared by 1 × TBST for 1 h at room temperature, and then incubated with the specific primary antibodies against NET1, p-ERK1/2, ERK1/2, VEGF and GAPDH (purchased from Cell Signaling Technologies, USA) respectively. Then membrane was then incubated with peroxidase-conjugated anti-rabbit or anti-goat IgG (purchased from ThermoFisher). These protein bands were visualized by adding ECL solution droply (Amersham Biosciences).
RNA extraction and qRT-PCR
TRIzol reagent (ThermoFisher) was used to extract total RNA from HCC cell lines or tissues according to the manufacturer’s user guidelines. Reverse transcription (RT) and one-step RT-PCR kit (Takara) were used to synthesize the first strand of cDNA. qRT-PCR was performed by SYBR Green and ABI apparatus (Thermofisher) according to the principles of manufactures. The relative level of gene expression was calculated by the 2−∆∆Ct method. Primers forward and reverse are chemically synthesized as follows: NET1, 5′-CTG TTC ACC TCG GGA CAT TT-3′ and 5′-TGG AGC TGT CAG ACG TTT TG-3′, GAPDH (5′-GGTATCGTGGAAGGACTCATGAC-3′ and 5′-ATGCCAGTGAGCTTCCCGT TCAGC-3′).
MTT assay
MTT was used to detect the effects of propofol on SMMC-7721 cell lines. Cells treated with the indicated propofol at doses of 0, 10, 25, 50, 100 μM for 48 h, and then incubated with MTT at room temperature for 4 h to produce formazan. Then, SDS-HCl was used to dissolve the formazan, and the absorbance at 570 nm was measured with a Universal Microplate Reader (Thermo). The cell viability was calculated by the following formula: Value of OD = OD of propofol-treated group/OD of blank control group.
IC50 values were the dose required to propofol inhibitory activity of 50% of the cell population and calculated from logarithmic sigmoidal dose–response curves generated using GraphPad Prism 5.0 software (GraphPad Inc).
Scratch wound assay
SMMC-7721 cells treated with or without propofol were seeded on 60 mm tissue-culture plastic dishes. When cells reached 80% cell confluence, a scratch wound was created using a sterile pipette tip. At 12, 24 and 48 h post-wound scratching, the cells among different groups were stained with 0.1% Crystal Violet and photographed in the same field of view. Wound closure was calculated according to the ratio of areas uncovered by cells before and after wound scratching.
Invasion assay
24-well plate of BD transwells was utilized to perform invasion assay. In brief, 5 × 104 cells in 100 μl serum-free medium were seeded on the upper chamber of matrigel-coated transwell, which were embedded into medium of the lower chamber. Six hundred μL 10% FBS serum medium was added to the lower chamber as a chemoattractant. After 48 h incubation, non-migrating cells were removed by wiping the upper chamber with a cotton swab. Migrated cells on the bottom side of the well were stained with Giemsa, and were counted in five random fields under a microscope (Olympus, Japan) at 40 × magnification.
Statistical analysis
Each assay was repeated at least in triplicates. Data were scientifically analyzed by Microsoft Excel and Graphpad Prism software, and presented as mean ± SEM. The intensity of western blot bands was measured by Image J software. Unpaired t test or one-way ANOVA was used to determine significant differences between two groups or multiple groups respectively.
Source: Ecology - nature.com


