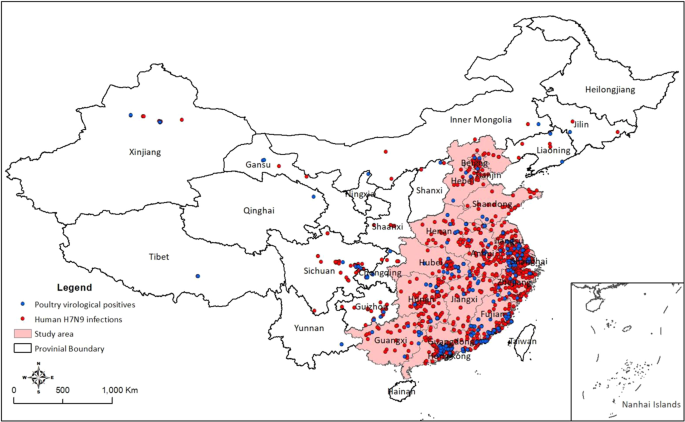
This study extends current knowledge3,22,23,24,25,26 about the spatiotemporal epidemiology of human H7N9 infections in a number of ways. Firstly, using the most complete data on human H7N9 infections and poultry LBM surveillance from 2013–2017, our spatial analyses mapped the spatial distribution of human H7N9 infections and its relationship with poultry serological and virological surveillance results. Second, our human H7N9 relative risk map displayed the distribution of high-risk areas associated with poultry infection status in the county, presence of wholesale LBMs, density of retail LBMs, human population density, chicken density and poultry movement network in the county.
Our analysis identified temporal lags between human H7N9 notifications and poultry surveillance recorded during 2013 to 2017. From examining the temporal relationship between human H7N9 infections and poultry H7N9 surveillance results, we detected a one/two-month temporal lag between the onset of human H7N9 infections and poultry virological/serological surveillance results. These temporal lags may be explained by, firstly, the sensitivity of serological surveillance for H7N9 in poultry is much higher than virological surveillance, and LPAI virus or its genome can be detected in an individual bird for only a few days due to the short period of virus shedding, whereas antibodies elicited by LPAI virus are often present for the entire production life of the infected poultry27,28. Meanwhile, due to low sensitivity, virological positives will be more likely to be detected when the concentration of virus has built up to a more detectable level most likely through the live poultry market chain, i.e. from farms then going through traders, wholesale markets and retail markets. Besides, our results demonstrated that most of the H7N9 virological positive samples were collected in LBMs10, which is consistent with the consensus that the primary risk factor for human H7N9 infections in China is exposure to LBMs11,12,13,14,15,16,17. These findings are also consistent with those from our spatial models of human H7N9 incidence, suggesting that the county-level incidence of human H7N9 infections is positively associated with the presence of poultry virological positives in the county. Together these findings have important operational implications for anticipating human H7N9 infections based on current routine LBM H7N9 surveillance in poultry.
Previous studies indicated that LBM density and the number of LBMs were important factors for explaining the risk of H7N9 human infections3,13,22,23,25,26,29. In our analysis, both the presence of wholesale LBMs and density of retail LBMs were positively associated with higher relative risk of human H7N9 infections. Wholesale LBMs bring together live birds from large catchment areas and birds are commonly traded to retail LBMs30,31; this results in market networks with numerous trade connections. Higher densities of markets may exacerbate that risk and explain the strong spatial correlation with suitability for H7N9 infection26. Closing LBMs appears to be an effective approach for eradicating or reducing H7N9 infections in humans32. However, a recent study presented evidence that the closure of LBMs in early waves of H7N9 influenza had resulted in expansion of H7N9 infection to uninfected areas20. This implies closing LBMs is a long-term strategy that needs to be further evaluated. Our recent meta-analysis identified biosecurity measures that have been effective for controlling AI viruses at LBMs include smaller market size, selling single poultry species and separating different species, mandatory monthly rest days and bans on keeping live birds overnight, and sourcing poultry from local areas33. These identified characteristics of LBMs allow us to better target control efforts.
Furthermore, in our model we included estimates of live chicken movement from areas originally affected areas by H7N9 in Southeast China, which allowed us to evaluate the effect of live chicken movement from the primary high-risk area on the overall distribution of human H7N9 infections from 2013 to 2017. Our results indicate a positive relationship between human H7N9 incidence and poultry movement estimates (degree centrality) from our CAR model. A previous study of poultry market chains in South China also reported that LBMs where HPAIV H5N1 was isolated were associated with higher degree centrality34. Poultry network studies in Vietnam and South China revealed that live poultry traders tend to link poultry sources of similar infection status34,35. These findings suggest that poultry movements from the originally affected area in east China provinces may continue to play a role in disseminating H7N9 virus throughout China. This further demonstrates the importance of evaluating live poultry movement and trading practices to develop appropriate and targeted surveillance recommendations for active H7N9 surveillance program.
After adjusting for poultry marketing system variables (presence of wholesale LBMs and density of retail LBMs) and spatial autocorrelation, our results indicated that human population density was negatively associated with the human H7N9 incidence while chicken density was positively associated with human H7N9 incidence. This can partly be explained by the known epidemiology of H7N9 in humans in that most human cases are a result of animal-to-human transmission, rather than human-to-human transmission. Since most H7N9 cases have been reported in large cities where human population density is very high, it may partially due to that the surveillance effort to detect H7N9 human cases was much greater in area with high population density and better medical facilities22,23. Moreover, higher human population density is usually related to higher biosecurity levels in the LBMs in highly dense urbanized areas. Furthermore, existing evidence indicates that H7N9 is more prevalent in chickens than in other poultry species3,8,36. Also, while H7N9 can affect other species it is mainly limited to chickens due the characteristics of the industry and the marketing system8. Higher chicken density is usually related to high chicken production, chicken trading and transportation which may promote transmission of the pathogen among poultry and increase the chance of humans acquiring H7N9 infection. Our findings suggest that highly connected areas with high chicken density and low human population should be targeted in case the virus continues to evolve or the efficacy of the vaccine is reduced, or even for the emergence of similar viruses in the future.
Moreover, the results of our study demonstrated significant spatial clustering of human H7N9 incidence in the study area, which required the development of a geographical model that incorporated spatial autocorrelation in order to generate a robust risk map of human H7N9 infection across China. Our human H7N9 relative risk map suggests that although H7N9 vaccine for poultry is currently available, continued active surveillance still needs to be strengthened for high-risk areas in China. Our results support strengthening LBM and human surveillance in Southeast area of China (involving Jiangsu, Zhejiang, Anhui provinces and Shanghai Municipality), coastal areas in Fujian and Guangdong provinces, and some inland areas in Hubei, Hunan and Guangxi provinces, as well as Beijing Municipality and the Northern area in Hebei province. According to the National Guidelines on the Prevention and Control of H7N9 influenza in Poultry in China (2018–2020)37, the current control of H7N9 infections in poultry in China has relied heavily on wide-scale compulsory preventive vaccination combined with biosecurity enhancement in both poultry farms and LBMs, regular surveillance programs, as well as live poultry movement control, quarantine and stamping out. The introduction of live poultry from high-risk areas and sites is strictly restricted37, however, the delimitation of high risk-areas is unclear. This study attempted a new risk assessment approach and the results provided recommendations to a more targeted risk-based surveillance program, as well as new insights into the role of LBMs and poultry movement in China. However, the map of the spatially structured random effects demonstrates evidence of clustering around the Yangtze River delta area, suggesting that there are other risk factors not included in our spatial models, such as people’s behaviour, or indeed other environmental factors that could account for the residual spatial distribution.
The results of this study should be interpreted in light of some limitations. Our analyses were based on laboratory-confirmed cases of human H7N9 infections and reported poultry H7N9 virological surveillance results, and are therefore subject to reporting bias, especially in areas of China with poor surveillance system coverage. In addition, our data for the distribution of LBMs were obtained from local veterinary departments except Shandong and Zhejiang provinces, data for these two provinces were replaced by another dataset clarified in the supplementary file, which may bring some reporting bias and uncertainty to the model. Furthermore, our live chicken movement data were collected in selected high-risk areas in Southeast provinces in 2014, representing the live chicken movements coming from and to the originally affected provinces, which may not reflect the current poultry movement situation across the region. The measures of degree centrality used in our model do not represent a perfect indicator of the “overtime” exposure of countries via movement of poultry from 2013 throughout 2017, its use in our model is important to consider in the context of the original source of the virus. We recognize that the effect of the measures of degree centrality are far from depicting a causal relationship and thus are prone to regression dilution bias; however, it is remarkable that despite this limitation we were able to identify a significant signal on the role of the poultry movements originating from the initially affected area.
In conclusion, contamination of LBMs with H7N9 is an important determinant of the risk of human H7N9 incidence in China. Moreover, poultry movement from the original areas of H7N9 emergence may be an important driver of the dissemination of H7N9 infections across China, and poultry serological positives and virological positives can serve as a predictor for human H7N9 infections as well as being a guide for the timing of risk management interventions. Highly connected areas with high chicken density and low human population should be targeted. It is recommended that regular monitoring of poultry movement and poultry infections at the high-risk counties identified in this study will provide essential evidence for the early warning of H7N9 infections across China.
Source: Ecology - nature.com


