The main differential morphological characters between the first protozoea stage of the two species are summarized in Table 1. Also, we propose an identification key to distinguish the first protozoeal stage of Dendrobranchiata larvae of species occurring in the Northeastern Atlantic ocean and Mediterranean Sea, gathering information from our own observations and from available literature31,32,33,34,35,36,37,38. The general body morphology description of the Dendrobranchiata first protozoea stage can be found in some recent references18,19. The first protozoea (PZ I) of Dendrobranchiata larvae has a carapace covering part of the cephalotorax, followed by an unsegmented pleon and finishing in a large bilobed telson (Figs. 1A, 2A, 3A, C–I, L). The carapace is unarmed in most of the Penaeidae (Figs. 1A, 2A, 3L) but the Solenoceridae (Fig. 3C), the Luciferidae (Fig. 3A) and the Sergestidae (Fig. 3D–I) possess dorsal and lateral spines or processes. The compound eyes are covered by the carapace (e.g. Fig. 3E), and the naupliar eye is still visible (Fig. 3C). These larvae have two pairs of antennae in the anterior part of the carapace: the first pair (antennula) is uniramous and the second one (antenna) is biramous. In the antennae (e.g. Fig. 3K, J, M), the exopod is composed by a long plumose outer ramus with several ringlets throughout its length, and the endopod is the inner ramus. The mouth appendices are composed by a pair of mandibles, with incisor and molar processes, and two pairs of maxillae. The larvae also present 2 pairs of biramous maxillipeds where the outer ramus is the exopod and the inner ramus is the endopod. The third pair of the maxilliped, when present, is still rudimentary.
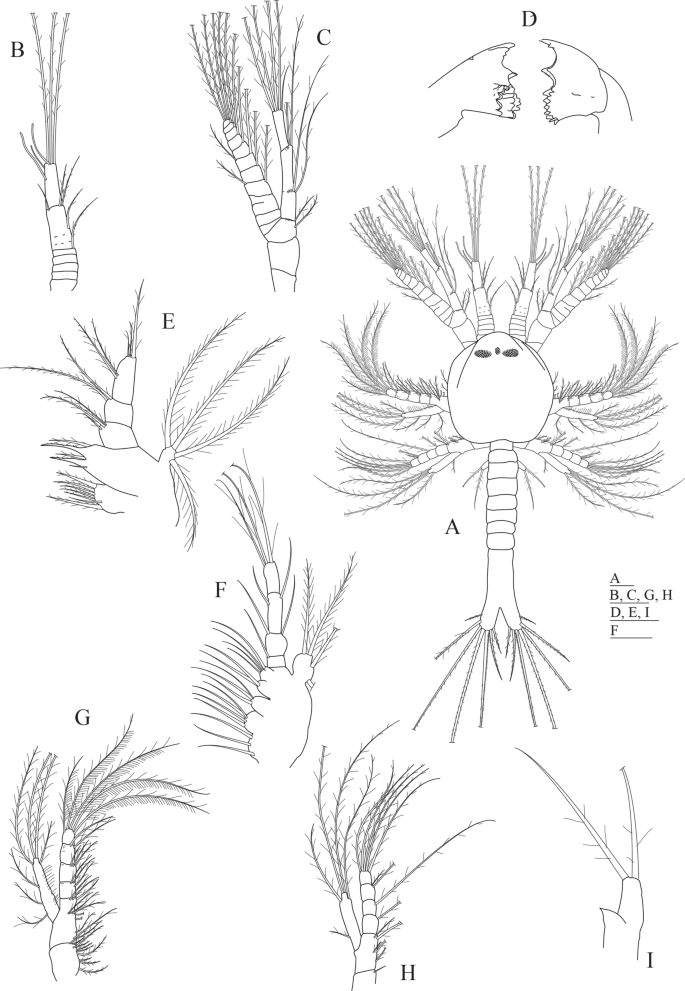
Aristeus antennatus first protozoea larva. (A) Dorsal view; (B) antennula; (C) antenna; (D) mandible; (E) maxillula; (F) maxilla; (G) first maxilliped; (H) second maxilliped; (I) third maxilliped. Drawn with GIMP software (v. 2.10.18, https://gimp.org).

Gennadas elegans first protozoea larva. (A) Dorsal view; (B) antennula; (C) antenna; (D) mandible; (E) maxillula; (F) maxilla; (G) first maxilliped; (H) second maxilliped; (I) third maxilliped. Drawn with GIMP software (v. 2.10.18, https://gimp.org).

Drawings of known protozoea I larvae of Dendobranchiata species. (A) Dorsal view of Lucifer penicillifer; (B) telson of Petalidium sp.; (C) dorsal view of Solenocera membranacea; (D) dorsal view of Parasergestes vigilax; (E) dorsal view of Sergestes atlanticus; (F) dorsal view of Eusergestes arcticus; (G) dorsal view of Deosergestes corniculum; (H) dorsal view of Sergia remipes; (I) dorsal view of Deosergestes henseni; (J) antenna of Penaeus kerathurus; (K) antenna of Penaeopsis rectacuta; (L) dorsal view of Sicyonia carinata; (M) antenna of Parapenaeus longirostris; (N) third maxilliped of Aristaeomorpha foliacea. All figures redrawn with GIMP software, (v. 2.10.18, https://gimp.org) from: A. 36; B and I. 27; C. 37; D, E, G and H. 38; F. 39; J, L and M. 5; K. 40; N. 20. Drawings not to scale.
Morphological description of Protozoea I of Aristeus antennatus (Fig. 1)
Size: TL (total length) = 1.12–1.25 mm; CL (carapace length) = 0.37–0.49 mm; N (number of protozoea examined) = 13.
Carapace (Fig. 1A): carapace almost rounded, longer than wider, reaching the level of the second maxilliped, with frontal organs visible at the anterior part; naupliar eye present flanked by a pair of compound eyes that are already visible through the carapace; 6 thoracic somites visible.
Antennula (Fig. 1A,B): first paired uniramous appendage in the cephalotorax, consisting of 3 articles: proximal article subdivided in 5 ringlets, bearing 1 short serrulate seta on the posterior end; second article with 1 positioned at mid-length of article and 3 serrulate setae distally; distal article with 3 aesthetascs subterminally and 3 long sparsely plumose setae on the posterior end.
Antenna (Fig. 1A,C): second paired biramous appendage in the cephalotorax, longer than antennula, consisting of a peduncle, an endopod and an exopod. Peduncle 3-segmented with 1 + 1 sparsely plumose setae on distal segment; endopod 2-segmented with 2 + 2 lateral plumose setae in proximal segment and 4 long plumose and 1 simple setae in the posterior segment; exopod with 11 ringlets, 3rd ringlet with a transversal incomplete separation, ringlets 4th to 11th each with a long plumose setae along inner margin and two more long plumose setae on the terminal position of the 11th ringlet, 4th and 6th ringlets each with an additional plumose setae on outer margin.
Mandible (Fig. 1D): the first paired appendage following the mouth placed in the ventral side of the cephalotorax, with distinct incisor and molar processes; incisive part with 2 + 2 teeth (the one placed near the molar part is short serrated); molar part with numerous small conate and uncinate teeth; without palp.
Maxillula (Fig. 1E): the second paired appendage following the mouth placed in the ventral side of the cephalotorax, divided in coxa, basipod, endopod and exopod. Coxa with 7 (1 simple and 6 papposerrate) setae; basipod with 5 (1 simple and 4 cuspidate) setae; endopod 3-segmented with 2 sparsely plumose and 1 small simple setae on the proximal segment, 2 sparsely plumose setae on second segment and 2 + 3 sparsely plumose setae on distal segment; exopod as a small knob-like structure with 4 long plumose setae.
Maxilla (Fig. 1F): the third paired appendage following the mouth placed in the ventral side of the cephalotorax, composed of coxa, basipod, endopod and scaphognathite. Coxal endite bilobed with 3 + 4 plumose setae; basial endite trilobed with 3 + 2 + 4 plumose setae; endopod 4-segmented bearing 1,1 + 1,1 + 1,3 long plumose setae; scaphognathite with 4 marginal long plumose setae.
First maxilliped (Fig. 1A,G): biramous paired appendage placed in the penultimate thoracic somite covered by the carapace, consisting of a protopod, an endopod and an exopod. Protopod 2-segmented (coxa and basipod), proximal coxal portion with 10 papposerrate setae; distal basial portion with 2 + 5 papposerrate setae along margin and 3 papposerrate seta on distal end; endopod 4-segmented with 3, 3, 2, 5 papposerrate setae; exopod unsegmented with 3 long and 4 plumose setae on distal margin.
Second maxilliped (Fig. 1A,H): biramous paired appendage placed in the last thoracic somite covered by the carapace, divided in coxa, basipod, endopod and exopod. Coxa with 1 seta; basipod with 1 + 2 + 2 papposerrate setae; endopod 4-segmented with 1, 1, 2, 4 papposerrate setae; exopod unsegmented with 1 + 4 long plumose setae.
Third maxilliped (Fig. 1A,I): biramous paired appendage placed in the first thoracic somite not covered by the carapace, consisting of an endopod and an exopod. Endopod represented by a small bud tapered at the end; exopod unsegmented with 2 long plumose setae distally.
Pereiopods: absent.
Pleon (Fig. 1A): pleomeres not completely differentiated, united with the telson and unarmed.
Pleopods: absent.
Uropods: absent.
Telson (Fig. 1A): broadly bifurcate with two distinct branches, each branch with 7 long plumose spines except the outermost one that is simple.
Morphological description of Protozoea I of Gennadas elegans (Fig. 2)
Size: TL = 0.86–1.22 mm; CL = 0.33–0.44 mm; N = 9.
Carapace (Fig. 2A): carapace almost rounded, longer than wider, reaching the level of the second maxilliped, with frontal organs visible at the anterior part; naupliar eye present flanked by a pair of compound eyes that are already visible through the carapace; 6 thoracic somites visible and not covered by the carapace.
Antennula (Fig. 2A,B): first paired uniramous appendage in the cephalotorax, consisting of 3 articles: proximal article subdivided in 5 ringlets; second article with 1 very small simple spine distally; distal article with 3 aesthetascs and 1 sparsely plumose setae subterminally and 3 long sparsely plumose setae on the distal end.
Antenna (Fig. 2A,C): second paired biramous appendage in the cephalotorax, longer than antennula, consisting of a peduncle, an endopod and an exopod. Peduncle 3-segmented with 2 sparsely plumose setae on distal segment; endopod 2-segmented with 2 + 2 lateral plumose setae in proximal segment and 4 long + 1 short plumose setae in the distal somite; exopod with 11 ringlets, ringlets 4th to 11th each with a long plumose setae along inner margin and two more long plumose setae on the terminal position of the 11th ringlet, 4th and 6th ringlet each with an additional plumose setae on outer margin.
Mandible (Fig. 2D): the first paired appendage following the mouth placed in the ventral side of the cephalotorax, with distinct incisor and molar processes; incisive part with 3 (one minute) + 2 (the one placed near the molar part is serrated) teeth; molar part with numerous small connate and uncinated teeth; without palp.
Maxillula (Fig. 2E): the second paired appendage following the mouth placed in the ventral side of the cephalotorax, divided in coxa, basipod, endopod and exopod. Coxa with 7 (1 simple and 6 papposerrate) setae; basipod with 4 (2 cuspidate and 2 papposerrate) setae; endopod 3-segmented with 2 sparsely plumose setae on the proximal segment, 2 sparsely plumose setae on second somite and 2 + 3 sparsely plumose setae on distal segment; exopod as a small knob-like structure with 4 long plumose setae.
Maxilla (Fig. 2F): the third paired appendage following the mouth placed in the ventral side of the cephalotorax, composed of coxa, basipod, endopod and scaphognathite. Coxal endite bilobed with 7 (1 small simple) + 2 plumose setae; basial endite trilobed with 5 + 4 + 3 plumose setae; endopod 2-segmented bearing 2 + 2 + 2, 3 long plumose setae; scaphognathite with 5 marginal long plumose setae.
First maxilliped (Fig. 2A,G): biramous paired appendage placed in the penultimate thoracic somite covered by the carapace, consisting of a protopod, an endopod and an exopod. Protopod 2-segmented (coxa and basipod), proximal coxal portion with 7 papposerrate setae; distal basial portion with 1 + 3 papposerrate setae; endopod 4-segmented with 2, 1, 2, 4 papposerrate setae; exopod 2-segmented with 1 + 1 + 1 + 2 setae along margin of proximal segment and 2 plumose setae on distal margin of terminal segment.
Second maxilliped (Fig. 2A,H): biramous paired appendage placed in the last thoracic somite covered by the carapace, divided in coxa, basipod, endopod and exopod. Coxa with 1 papposerrate seta; basipod with 1 + 2 + 2 papposerrate setae; endopod 4-segmented with 2, 1, 2, 4 papposerrate setae; exopod unsegmented with 1 + 1 + 2 + 2 long plumose setae.
Third maxilliped (Fig. 2A,I): biramous paired appendage placed in the first thoracic somite not covered by the carapace, consisting of an endopod and an exopod. Endopod represented by a small bud rounded at the end; exopod unsegmented with 2 long plumose setae distally.
Pereiopods: absent.
Pleon (Fig. 2A): 2 pleomeres differentiated, all the others united with the telson and unarmed.
Pleopods: absent.
Uropods: absent.
Telson (Fig. 2A): broadly bifurcate with two distinct branches, each branch with 7 long plumose spines.
Identification key for the first protozoeal stage of Dendrobranchiata larvae of the Northeastern Atlantic and Mediterranean Sea
| 1 | Rostrum present (Fig. 3A) | 2 |
| Rostrum absent (Fig. 3C–I, L) | 3 | |
| 2 | Telson with 5 pairs of spines (Fig. 3A) | Lucifer and Belzebub |
| Telson with 6 pairs of spines (Fig. 3B) | Petalidium | |
| 3 | Pereion margin with spines or processes (Fig. 3C–I) | 4 |
| Pereion margin smooth (Fig. 3L) | 10 | |
| 4 | Pereion octagonal with a pair of robust spines at each vertice (Fig. 3C) | Solenocera membranacea |
| Pereion with anterior, lateral and posterior processes (Fig. 3D–I) | 5 | |
| 5 | Pereion anterior process with 3 branches (Fig. 3D–F) | 6 |
| Pereion anterior process with 4 branches (Fig. 3G–I) | 8 | |
| 6 | Median branch of the anterior process of pereion with denticles only (Fig. 3D) | Parasergestes vigilax |
| All branches of anterior pereion process with denticles (Fig. 3E, F) | 7 | |
| 7 | Telson branches long and narrow, length more than 3 times the width (Fig. 3E) | Sergestes atlanticus |
| Telson branches short, length only slightly greater than width (Fig. 3F) | Eusergestes arcticus | |
| 8 | Posterior process of pereion swollen at base (Fig. 3G) | Deosergestes corniculum |
| Posterior process of pereion not swollen at base | 9 | |
| 9 | Lateral process with 7 long spines at the base (Fig. 3H) | Sergia remipes |
| Lateral process with 3 long spines at the base (Fig. 3I) | Deosergestes henseni | |
| 10 | Setal formula of antennal protopod and endopod is 1,1,2, third maxilliped absent (Fig. 3J) | Penaeus (Melicertus) kerathurus |
| Setal formula of antennal protopod and endopod is 1,2,2 (Fig. 3K) | Penaeopsis | |
| Setal formula of antennal protopod and endopod is 1,2,3 (Fig. 3M) | 11 | |
| Setal formula of antennal protopod and endopod is 2,2,2 (Figs. 1C, 2C) | 12 | |
| 11 | Length of antennula 2 × longer than antenna (Fig. 3L) | Sicyonia carinata |
| Length of antennula approximately equal to that of antenna | Parapenaeus longirostris | |
| 12 | Exopod of the third maxilliped with 3 setae (Fig. 3N) | Aristaeomorpha foliacea |
| Exopod of the third maxilliped with 2 setae (Figs. 1I, 2I) | 13 | |
| 13 | Setal formula of antennula is 0,1,4 (Fig. 2B) | Gennadas elegans |
| Setal formula of antennula is 1,4,3 (Fig. 1B) | Aristeus antennatus |
Discussion
Although morphologically quite similar in most of their characters, the first protozoeal stages of A. antennatus and G. elegans bear some differences that will allow to distinguish them, as shown in Table 1 and in the identification key proposed. The first protozoea of A. antennatus presents 1, 4, 3 setae along the segments of the antennula, whereas in the case of G. elegans, the setal formula is 0, 1, 4. These characters are relatively easy to observe at the stereomicroscope, in most cases without the need of dissecting the specimens, and should provide an easy guide to differentiating the first protozoea of these two species.
The identification and morphological description of the larval series of A. antennatus found in the plankton off the Balearic archipelago by Heldt in 195520 has proven to be fundamentally correct, as the descriptions of the rest of known stages of the species—PZ II, PZ III and mysis I—have been recently confirmed22. However, when comparing the A. antennatus PZ I from the present study with the one described by Heldt20, we found differences in the size of the larvae—the sole specimen in the cited study measured 1.55 mm, whereas in the present study the average total length is 1.2 mm. Moreover, we found differences between the two studies in the number of aesthetascs on the antennula, and in the number of setae on the exopod of the third maxilliped. While the possibility of an error can never be excluded, Heldt’s meticulous work and thorough descriptions in all her publications on Penaeid larvae make it unlikely that she would draw and describe a morphological character that she did not observe. We here expose our considerations about this contradiction.
First, Heldt’s study refers that one single specimen of first protozoea stage was caught for each of the studied species, A. antennatus and A. foliacea, but that the latter was apparently lost during manipulation and could not be described. Second, as seen in Table 1, the total length of the A. antennatus PZ I specimen measured by Heldt is 1.55 mm, while the next stage, PZ II, measured 1.50–2.03 mm20: this would mean that the PZ II was smaller than its previous stage. Variability in total length of these larvae has not been studied and might allow for such values, but Carreton et al.22 found an average total length of only 1.2 mm (± 0.05) for the PZ I. On the other hand, the PZ II of A. foliacea examined by Heldt measured 1.9 mm20 which is more in agreement with the length of the PZ I larva described as A. antennatus. Finally, Heldt’s description of A. antennatus PZ I accounts for 3 setae on the exopod of the third maxilliped (mxp3), whereas in our findings, all individuals presented only 2 setae. Furthermore, it seems that, in Heldt’s description, A. foliacea PZ II larvae present more developed characters than A. antennatus PZ II, as the mxp3 is described in A. foliacea with 3 setae on the exopod and 2 on the endopod, while in the case of A. antennatus, it only presents setae on the exopod. It would then be possible that, in the case of the PZ I, the more setose (3-setae) third maxilliped belongs to A. foliacea and the less setose (2-setae) one belongs to A. antennatus. For these reasons, we conclude that Heldt’s description of A. antennatus PZ I is probably that of A. foliacea. The PZ I of A. antennatus would then have remained undescribed until now.
The present study provides the first detailed morphological description of the protozoea I larvae of A. antennatus and G. elegans according to modern standards, made from plankton samples after identification being confirmed with molecular analysis. The protozoea I larvae of the two studied species can be morphologically distinguished from one another mainly by the setation of the antennula. An identification key is provided allowing for the morphological identification of all first protozoea larvae of Dendrobranchiata for the Mediterranean Sea and Northeast Atlantic Ocean known today.
In a context where fisheries science is increasingly drawing on marine connectivity to design regional-scale management strategies for commercial species, larval distribution studies are one of the first stepping stones to effective planning, as they broaden the knowledge on species dispersal patterns. It is then essential to ensure a correct identification of these larvae, and morphological characters provide accurate, at-hand information even when molecular methods are not applicable. Our results set a starting point for A. antennatus connectivity studies in the frame of fisheries management, and we are confident that the identification key provided will make classification of the featured early larval stages accessible to both taxonomers in the field and non-specialists.
Source: Ecology - nature.com


